Ectodomain Shedding and Enrichment of the Soluble Membrane Proteome
Background
The human genome encodes for about 15,000 transmembrane proteins, encompassing 19% of the total genome. Through a regulated proteolytic mechanism called ectodomain shedding, the extracellular domains of membrane-anchored proteins are sometimes released from the cell surface as soluble proteins. During the shedding process, a protease (referred to as sheddase) cleaves a membrane protein substrate close to or within its transmembrane (TM) domain, resulting in release of the soluble extracellular domain (ectodomain) from the membrane and a fragment that remains bound to the membrane (Figure). Sheddases and their transmembrane protein substrates are known to be involved in many diseases; an example is the excessive level of shed TNFα, which is implicated in numerous inflammatory conditions, including sepsis, rheumatoid arthritis, and lupus1. Therefore, proteomic profiling of the soluble ectodomain sub-proteome provides opportunities to discover important drug targets or personalized biomarkers.
Cells use ectodomain shedding to regulate the expression and function of surface molecules, and modulate a wide variety of cellular and physiological processes. This mechanism is important in a variety of normal and pathological processes, including growth factor signaling, cell adhesion, inflammation and proliferation. Thus, ectodomain shedding is a process that is highly regulated by specific agonists, antagonists, and intracellular signaling pathways. As a result, any dysregulation within shedding processes can result in diverse pathologies such as inflammatory lung injury, infection, cancer, and Alzheimer’s disease2.
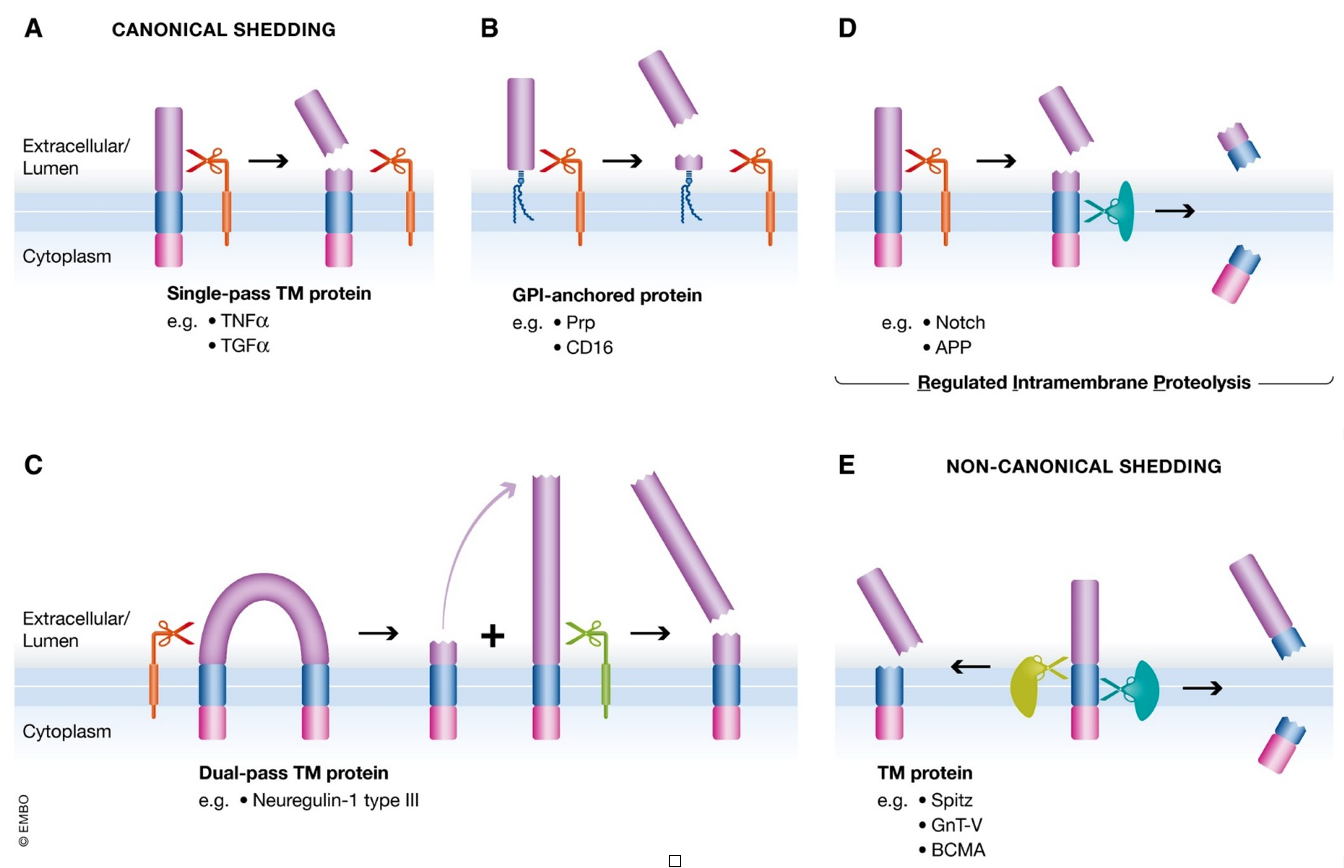
Challenge
Soluble membrane protein biomarkers that can be reported from biofluids, especially from blood/plasma/serum is challenging for several reasons. First is dynamic range, as high abundance proteins can mask potentially significant biomarker proteins of low-abundance. Another challenge is presented in targeted proteomic workflows, whereby a selection of biomarkers of interest is known, but lack efficiency and consistency in their measurements from different sample cohorts. This is in part due to the changing landscape of proteins/peptides not associated with the selected biomarkers, which can affect both ion suppression and spectral overlap from co-eluting off-target peptides. Both of these challenges are addressed by the NRicher™ suite of products.
Solution
For over 10 years BSG has been at the forefront of developing synthetic beads (i.e., ionic, hydrophobic, hydrogen bonding, aromatic, polymeric) with differential proteome binding properties.
The NRicher™ Advantage
Not derived from immuno-affinity: NRicher™ beads, are not species-specific. This allows a wider applicability across various sample types.
Cost-efficient:
There's no need for an investment in high-end specialized equipment; a standard laboratory microfuge will suffice.
Streamlined Analysis:
Through the use of bead cocktails, NRicher™ products serve virtually all applications, starting with sample volumes as low as 25 µl.
On-bead digestion:
BSG pioneered Bead-Assisted Sample Prep (BASP™), offering workflow efficiencies (i.e., no added denaturants) for LC-MS proteomics of bead enriched sub-proteomes.
The
NRicher™, product line continues to advance sub-proteome enrichment, with products tailored to specific applications. NRicher™ Mx is a particularly powerful tool for enriching low abundance proteins and especially soluble membrane proteins, which play critical roles in cellular processes. Here is a representative partial list of soluble membrane proteins observed after NRicher™ Mx bead enrichment from pooled normal human serum, only six of which are observed in neat (non-enriched) serum under the same LC-MS conditions.
Three proteins from this Gene List above, are especially noteworthy as they have been reported as soluble biomarkers in a variety of disease states.
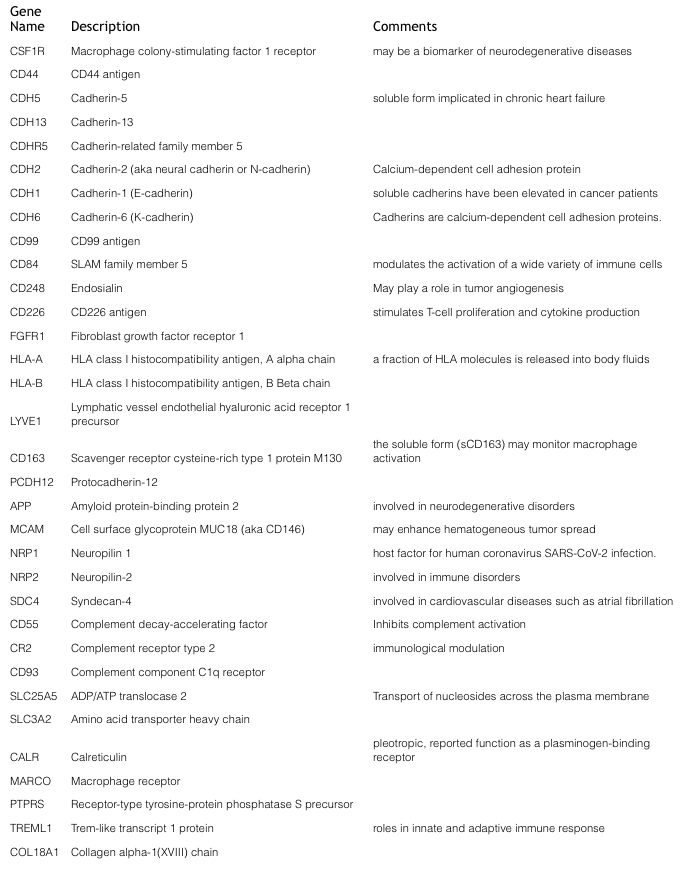
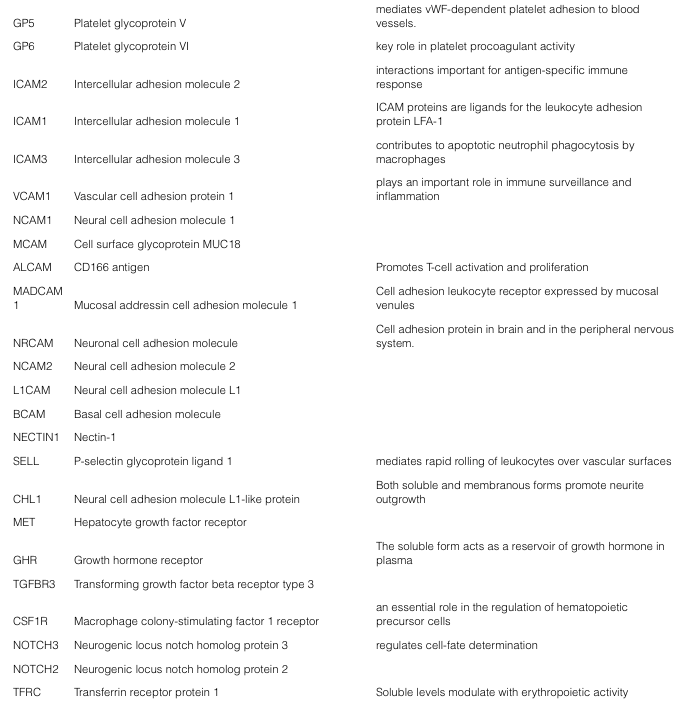
CD55 (aka Decay-accelerating factor or DAF)
At the convergence between the adaptive and innate immune response is the complement system—an evolutionarily conserved response that aids in the destruction of microorganisms and impaired cells. Because excessive activation of complement leads to unwanted cellular damage, tight regulation is necessary, and is managed by membrane proteins such as CD55, and soluble proteins including factor H, C1-inhibitor, C4b-binding protein, clusterin, and vitronectin. Decay-accelerating factor (DAF or CD55) plays a role in various diseases; as a positive regulator of tumorigenesis and malarial infection, but also as a negative regulator of paroxysmal nocturnal hemoglobinuria, MS, and autoimmune diseases3. Furthermore, there have been reports of soluble CD55 (sCD55) in a range of chronic inflammatory conditions, including rheumatoid arthritis, inflammatory bowel disease and several cancers. To better understand the seemingly contradictory roles of CD55 for each disease, targeted quantitative proteomic investigations is warranted.
CDH1 (E-cadherin) & CDH2 (N-cadherin)
In tumors, epithelial–mesenchymal transition (EMT) describes the transformation of tumor cells from a non-motile epithelial phenotype to a migratory mesenchymal phenotype. A common characteristic of EMT is the loss of epithelial calmodulin (E-cadherin) expression, which is accompanied by the upregulation of N-cadherin expression; a so-called “cadherin switch”. This change in cadherin expression increases the ability of tumor cells to invade and metastasize to distant sites, predicating poor prognosis. Proteolytic shedding of the extracellular domain results in soluble cadherins, circulating in blood. Elevated levels of sCDH1, sCDH2 and other cadherins have been detected in cancer patient serum when compared with healthy persons4. Therefore, soluble cadherins might serve as important biomarkers for cancer metastasis and prognosis.
MCAM (aka CD146)
The plasma concentration of soluble CD146 (sCD146) is modulated in inflammatory diseases associated with endothelial alterations, including inflammatory bowel disease, chronic renal failure, chronic liver disease and cancer5,6. The transmembrane CD146 glycoprotein is expressed by a variety of cancer cell types and is correlated with poor cancer prognosis and tumor aggressiveness. Soluble CD146, was found to induce EMT, cancer stem cell generation, and tissue factor expression in cancer cells7.
Conclusions
These examples demonstrate how NRicher™ Mx has a unique affinity towards the low abundance soluble membrane proteome, and can advantageously serve the two main goals of proteomics:
- Discovery and coverage of sub-proteomes to identify and monitor the signal of low abundance protein(s) or their modifications, that associate with disease, and
- Targeted quantitative workflows, when the protein biomarkers of interest are known and enrichment increases efficiency and reproducibility of multiplex measurements.
The simplified NRicher™ workflows help to minimize technical variance or bias in the data, an important consideration when large cohorts are required to make conclusions from small but significant biological variance between samples. Technical variances can arise from LC co-eluting peptides that both suppress ion signals, and interfere with spectral identifier assignments. Using NRicher™, enrichment of low abundance protein targets (<1 µg/ml) reach MS signal levels typically observed for mid-abundance proteins (1-100 µg/ml) and with less off-target background noise, well suited for quantitation of target peptide signals. Consequently, NRicher™ sub-proteome enrichment can minimize acquisition time, collectively improving overall throughput, cost, and productivity. For these reasons, sample prep workflows combining NRicher™ sub-proteome enrichment with on-bead digestion is especially desirable. Supplied as dry powder, NRicher™ beads are applicable to a variety of scalable formats, for high-throughput and automation. Eluates from NRicher™ beads can also be used in orthogonal analyses, such as enzymatic/functional assays, 2DE, or ELISA/immunoassay to provide further validation of biomarkers. Finally, through a searchable knowledgebase of >2000 serum proteins, a selection of one or more NRicher™ bead(s) and/or our depletion products can be investigated for specific protein targets of interest.
For more information on the full line of NRicher™ products, go to:
Introducing NRicher™: Bridging the Gap in Low-Abundance Proteome Enrichment (biotechsupportgroup.com)
For more information on NRicher™ Mx, go to:
NRicher™ Mx (biotechsupportgroup.com)
Related Separations, Enrichment/Depletion & Sample Prep -
All Product Categories (https://www.biotechsupportgroup.com/Products-a-z_a/258.htm)
Albumin & IgG Removal (https://www.biotechsupportgroup.com/Articles.asp?ID=451)
Lipid Removal and Clarification (https://www.biotechsupportgroup.com/Articles.asp?ID=456)
Hemoglobin Removal (https://www.biotechsupportgroup.com/Articles.asp?ID=452)
Sample Prep – Liquid Biopsy (https://www.biotechsupportgroup.com/Articles.asp?ID=457)
Sample Prep – Glyco, Virus, Aqueous Protein Crash/Metabolomics (https://www.biotechsupportgroup.com/Articles.asp?ID=453)
References
1. Lichtenthaler, Stefan F., Marius K. Lemberg, and Regina Fluhrer. "Proteolytic ectodomain shedding of membrane proteins in mammals—hardware, concepts, and recent developments." The EMBO journal 37.15 (2018): e99456.
2. Hayashida, Kazutaka, et al. "Molecular and cellular mechanisms of ectodomain shedding." The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 293.6 (2010): 925-937.
3. Dho, So Hee, Jae Cheong Lim, and Lark Kyun Kim. "Beyond the role of CD55 as a complement component." Immune network 18.1 (2018).
4. Derycke, Lara, et al. "Soluble N‐cadherin in human biological fluids." International journal of cancer 119.12 (2006): 2895-2900.
5. Ilie, M., et al. "Clinical value of circulating endothelial cells and of soluble CD146 levels in patients undergoing surgery for non-small cell lung cancer." British journal of cancer 110.5 (2014): 1236-1243.
6. Efrossini Nomikou, Alexandra Alexopoulou, Larisa Vasilieva, Danai Agiasotelli, Efthimia Pavlou, George Theodossiades & Spyridon P. Dourakis (2015) Soluble CD146, a novel endothelial marker, is related to the severity of liver disease, Scandinavian Journal of Gastroenterology, 50:5, 577-583, DOI: 10.3109/00365521.2014.985706
7. Stalin, Jimmy, et al. "Therapeutic targeting of soluble CD146/MCAM with the M2J‐1 monoclonal antibody prevents metastasis development and procoagulant activity in CD146‐positive invasive tumors." International Journal of Cancer 147.6 (2020): 1666-1679.
|
|
|