BSG Products To Assist in Analyzing Macrophage Polarization
Background
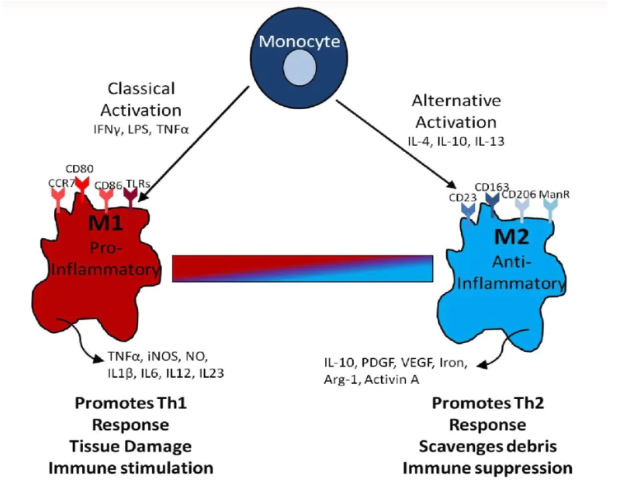
Macrophage polarization refers to how macrophages have been activated at a given point in space and time. Polarization is not fixed, as macrophages are sufficiently plastic to integrate multiple signals, such as those from microbe infections, damaged and cancerous tissues, as well as the normal tissue environment.
Today, the macrophage field has arrived at a partial consensus to describe the broad grouping of macrophage activation phenotypes. For example, M1 macrophages arise in inflammatory settings dominated by Toll-like receptor (TLR) and interferon signaling and are generally associated with immunity to bacteria and intracellular pathogens. M2 macrophages are found in settings dominated by TH2 responses.
Biochemical pathway regulation and output are integral to polarization. For example, fatty acid uptake and metabolism via CD36, lipoprotein lipase, and mitochondrial oxidative phosphorylation are required for M2 macrophage development and activation. By contrast, M1 macrophages are glycolytic. The varied effects of basic metabolic pathways on macrophage polarization are only just coming to the fore.
To summarize, polarization is dynamic across time and involves the tissue microenvironment.
Challenge
The need for new methods to model macrophage polarization helps to define mechanisms and potential biomarkers. Pro-inflammatory M1 macrophages (classically activated) and anti-inflammatory M2 macrophages (alternatively activated), are most often characterized based on several key surface markers and the production of inflammatory mediators. For example, M1 macrophages express CD80 and CD86, while M2 macrophages express CD163 and CD206 on the cell surface. Some of these are observable in biofluids, upon ectodomain shedding.
To study all facets of this, suitable products and methods are necessary.
Solution
As represented by these citations, Cleanascite™, our lipid removal product, is especially suited to study macrophage polarization in vitro.

Zhang, T., Zhao, F., Hu, Y. et al. Environmental monobutyl phthalate exposure promotes liver cancer via reprogrammed cholesterol metabolism and activation of the IRE1α-XBP1s pathway. Oncogene (2024). https://doi.org/10.1038/s41388-024-03086-1
Humans are widely exposed to phthalates, a major chemical plasticizer that accumulates in the liver. However, little is known about the impact of chronic phthalate exposure on liver cancer development. In this study, we applied a long-term cell culture model by treating the liver cancer cell HepG2 and normal hepatocyte L02 to environmental dosage of monobutyl phthalate (MBP), the main metabolite of phthalates. MBP exposure triggered the reprogramming of lipid metabolism, which contributed to an immunosuppressive tumor microenvironment (TME). Abnormalities in lipid metabolism is a molecular hallmark for not only cancer cells, but also tumor-associated immune cells.
To study those abnormalities, the article states “To investigate the role of lipids in regulating the macrophage function, we first employed Cleanascite to remove all lipids and lipoproteins from the conditioned media (CM) of HepG2 cells. Interestingly, lipid-depleted HepG2 CM significantly suppressed the migration ability of THP-1PMA cells both in control and MBP-exposed groups (Fig. D). Also, the expression of M2-type markers (such as CD206, CD163, and ARG1) in THP-1PMA cells dramatically decreased after lipid clearance, while the expression of M1-type markers (such as iNOS, CD80, and CD86) was up-regulated.”.
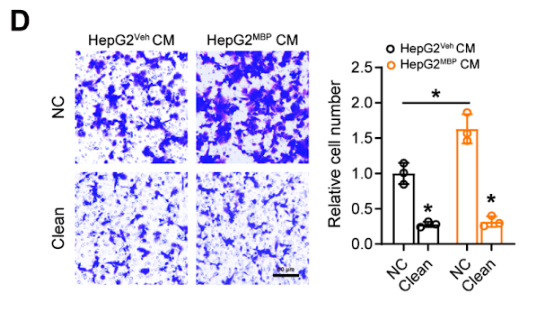
Figure S8. MBP-induced tumor cell cholesterol accumulation promotes macrophage infiltration and M2-polarization
N(D) Detection of THP-1PMA cell migration ability affected by conditioned medium (CM) from P30 HepG2MBP and HepG2Veh cells treated with or without Cleanascite (n = 3). Scale bar, 50 μm.
The authors conclude that MBP induced cholesterol accumulation in HepG2 cells, facilitated M2 polarization of macrophages, thereby contributing to an immunosuppressive Tumor microenvironment (TME) that further favored cancer development.
Chan, David, et al. "Polyunsaturated Fatty Acids Promote Protumoral Macrophage Polarization via a RhoA-YAP1 Signaling Pathway in the Ovarian Cancer Microenvironment." (2022).
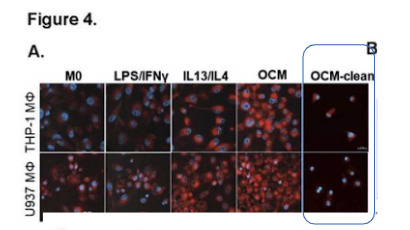
Tumor-associated macrophages (TAMs) are crucially associated with tumor development and progression; however, it remains unclear how the tumor microenvironment (TME) rewires the metabolic circuits and preferentially induces TAMs to polarize toward a protumoral phenotype. This study reports that polyunsaturated fatty acids (PUFAs) in malignant ascites promote protumoral M2-like TAMs deposition and facilitate peritoneal metastases of epithelial ovarian cancer (EOC). The article states “To selectively remove lipids, Cleanascite™ Lipid Removal Reagent (Biotech Support Group) was added to the omental conditioned medium (OCM) according to the manufacturer’s suggestions. … Intriguingly, the removal of free fatty acids in OCM by the Cleanascite™ attenuated lipid droplets deposition in M2-like MΦs and OCM-MΦs (Fig. 4A), indicating protumoral M2-like TAMs exhibited higher lipid accumulation and metabolism in the fatty acid-enriched OCM or the malignant ascites.”
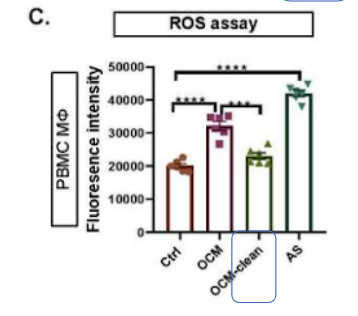
“… Similarly, we demonstrated that the cellular ROS levels in OCM/AS-MΦs derived from PBMC MΦs were significantly upregulated, whereas the addition of Cleanascite™ mitigated the increased malignant ascites microenvironment (MAM)-mediated ROS level, indicating that the accumulation of UFAs in the MAM is responsible for the enhanced ROS production in TAMs (Fig. 4C). … Here, we further found …removal of lipids by Cleanascite™ remarkably prevented the reduction of YAP1 in OCM-MΦs, implying that the lipids within MAM facilitates protumoral M2-like TAMs polarization through the regulation of RhoA-YAP1 signaling cascade.” The article concludes that PUFAs are a key player in promoting tumor-infiltrated TAMs polarization that, in turn, facilitates EOC tumor growth and metastasis.

Di Conza, Giusy, et al. "Tumor-induced reshuffling of lipid composition on the endoplasmic reticulum membrane sustains macrophage survival and pro-tumorigenic activity." Nature immunology (2021): 1-13. Tumor-associated macrophages (TAMs) display pro-tumorigenic phenotypes for supporting tumor progression in response to microenvironmental cues imposed by tumor and stromal cells. However, the underlying mechanisms by which tumor cells instruct TAM behavior remain elusive. The investigators here uncover that tumor-cell-derived glucosylceramide stimulated unconventional endoplasmic reticulum (ER) stress responses by inducing reshuffling of lipid composition and saturation on the ER membrane in macrophages.
As part of the study, a comparison of culture media (CM) with and without lipids was made. The article states “To generate culture media without lipids, …CM was treated with Cleanascite according to the manufacturer’s instructions.”. The authors uncover the unexpected roles of tumor-cell-produced lipids that simultaneously orchestrate macrophage polarization and survival in tumors via induction of ER stress responses and reveal therapeutic targets for sustaining host antitumor immunity.
For removal of Hemoglobin interference, the following citation is provide.
 The citation is:
The citation is:
Zhang, X., Li, S., Malik, I. et al. Reprogramming tumour-associated macrophages to outcompete cancer cells. Nature (2023). https://doi.org/10.1038/s41586-023-06256-5
Tumours are metabolically active and are populated by stroma cells, but how environmental factors affect cancer cell competition remains largely unknown. The study evaluated how tumour-associated macrophages (TAMs) can be dietarily or genetically reprogrammed to outcompete MYC-overexpressing cancer cells. In a mouse model of breast cancer, MYC overexpression resulted in an mTORC1-dependent ‘winner’ cancer cell state. To measure the amino acid content by LC-MS from tumor interstitial fluid, the article states “Samples were brought through a NuGel-HemogloBind (Biotech Support Group) prep prior to extraction to remedy the levels of haemolysis present. The article concludes noncanonical engulfment-medieated Rag GTPase-independent mTORC1 signalling in TAMs controls competition between TAMs and cancer cells, which defines a novel innate immune tumour suppression pathway that could be targeted for cancer therapy.
Macrophage biomarkers in biofluids
For macrophage biomarkers that can be observed in plasma, we can turn to
NRicher™ knowledgebase. The NRicher™ Knowledgebase & Supporting Products/Methods provides a database for researchers to find their protein(s) of interest, and determine the NRicher™ bead/methods to best enrich for those targets. Its free to review, and accessible on a non-confidential basis. Over 2000 proteins, from a pooled normal/healthy population are annotated with corresponding relative signal intensities for each bead/method. Additional annotations include curated prospective biomarkers, soluble membrane proteins, and the interconnected sub-proteomes of innate immunity. NRicher™ target(s) enrichment can minimize acquisition time, collectively improving overall throughput, with outstanding gains in productivity. NRicher™ methods offer scalability, automation compatibility, and cost-effectiveness, without any upfront instrument requirements.
For Macrophage biomarkers, we highlight:
Gene Name: CD14
Uniprot Identifier: P08571
Gene Description: Monocyte differentiation antigen CD14 (soluble form aka presepsin)
Soluble CD14 (sCD14) is a co-receptor for lipopolysaccharide (LPS) that is released from monocytes upon activation. Monocyte-macrophage activation markers, sCD14 & sCD163 are increased in SARS-Cov-2 infection.
Gene Name: CD163
Uniprot Identifier: Q86VB7
Gene Description: Scavenger receptor cysteine-rich type 1 protein M130
Gene Name: MRC1 (aka CD206)
Uniprot Identifier: P22897
Gene Description: Macrophage mannose receptor 1 (aka mannose receptor)
CD163, a hemoglobin-haptoglobin scavenger receptor, is exclusively expressed by monocytes and macrophages whose main biological function is the elimination of hemoglobin-haptoglobin complexes during hemolysis. The mannose receptor (CD206) is primarily expressed by M2-like macrophages, dendritic cells and endothelial cells. Both CD163 and Mannose Receptor exist as soluble serum proteins (sCD163 and sMR/sCD206) that may reflect the M2-like state of tissue macrophages, including Tumor-associated macrophages (TAMs).
For more information:
Click to visit Cleanascite™ product page and ordering information
Click here for more information on NuGel™ HemogloBind™
Click here for more information on all of our Hemoglobin Removal products
For more information on the NRicher™ platform, visit: https://www.biotechsupportgroup.com/NRicher-s/329.htm
For more information on the NRicher™ Knowledgebase, visit: https://www.biotechsupportgroup.com/category-s/333.htm
|
|
|