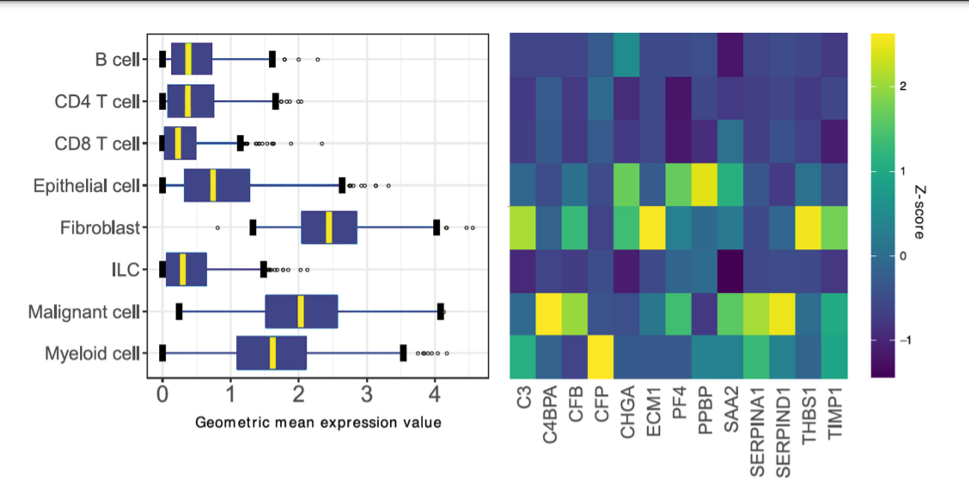
The Stromal-Epithelial Gene Signature Ratio, is based on genes from the SLB panel, and classified by their expression as being derived from stromal-phenotypic or epithelial-phenotypic cells. In this way, it is demonstrated that histologic high stromal content is accompanied by increased gene expression of stroma-associated pathways in comparison to tumors with low stromal content. The gene signature ratio described in this study was found to be related to previous investigations of TSR and demonstrated a remarkably similar prognostic performance. These findings provide further molecular evidence for the prognostic power of the tumor stroma in clinical practice.
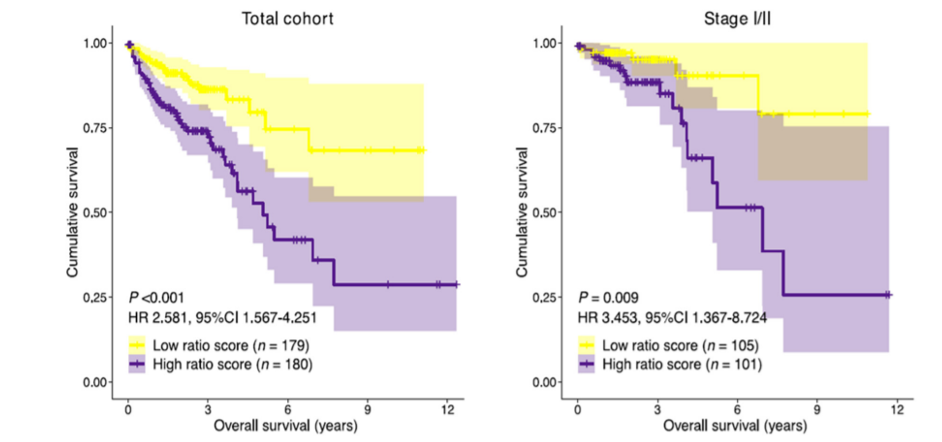
In addition to patient prognosis, high gene signature ratio-risk scores were associated with an increased proportion of Microsatellite instability (MSI) in comparison to low ratio-risk scores. Given the increased proportion of MSI in the high ratio risk score group, the signature ratio might be predictive of immune checkpoint inhibitor (ICI) therapy response in colon cancer and should be the subject of future studies in ICI therapy-treated patient cohorts.
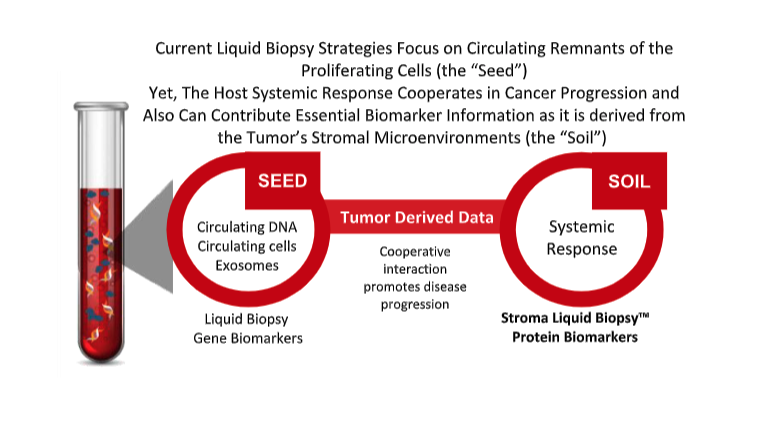
The current report provides a first theoretical framework for proteomic signatures to potentially serve as an indicator for tumor-stroma content when applied in liquid biopsy. Ultimately, the stromal conditioning protein blueprint, as captured by the SLB panel, may provide a more refined stratification of the tumor and patient prognosis, and offer new insights into therapeutic strategies that might beneficially modulate the tumor-microenvironment.
Finally, our selection of patent pending Stroma Liquid Biopsy™ biomarkers offer key analytical benefits as they are:
• With few exceptions, of relative high abundance in serum and measurable by LC-MS
• all highly differentiated – many severely, in the cancer population, and very stable in the normal/healthy population
• pleotropic and determinately linked to innate immunity
• in part, functional sub-forms, that can now be monitored by our patent pending methods, and which cannot be monitored by antigen presentation, aptamer or like binding motifs.
“It is very rewarding to see how, through the use of one of our enrichment products - AlbuVoid™, because of its unique selection properties, we observed something that others did not. Our experimental design was at first quite simple. Using proteomic data, we wanted to determine whether a systemic response was measurable in blood, to most if not all cancers, regardless of primary tumor, stage, or metastatic disease. This discovery research ultimately led to our patent pending panel of Stroma Liquid Biopsy™ biomarkers. From this, the genes from this panel have now been investigated through our collaboration with Leiden, providing a first theoretical framework for the panel’s suitability in liquid biopsy. We welcome commercial partnership opportunities so that we can advance the clinical utility for proteomic characterization of stromal conditioning in cancer. This can be an objective way to stratify patients towards the best treatment options, and personalize bedside decisions, which ultimately can prolong survival.” Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Lead authors Dr.Wilma Mesker (Associate Professor) and Cor Ravensbergen (Senior Medical Student) of the Leiden University Medical Center concur, and state further that, “the tumor-stroma microenvironment is an important prognostic parameter for patients with epithelial cancer types. However, tissue staining provides only qualitative information and does not offer any insight into specific cellular or protein mechanisms that impact survival. We do know that patients with a high amount of stromal cells in the primary tumor have a bad prognosis and respond worse to current chemo-, radio- and/or immunotherapy regimens. Now with the help of BSG’s Stroma Liquid Biopsy™ panel, our working hypothesis is showing real evidence for how stromal conditioning impacts survival. We will soon start our LC-MS analysis of the Stroma Liquid Biopsy™ protein panel on patient sera. From this analysis, we can envision future therapeutic strategies that can potentially modulate the tumor microenvironment, as the tumor-stroma ratio was found to be associated with pathologic response to neoadjuvant therapy. This supports the notion that the tumor microenvironment affects therapeutic response. So, we can begin to think about how modulation of stromal conditioning might improve immuno-oncology treatments, for example turning ‘cold’ tumors to ‘hot’. Consequently, we are very excited about the prospects for bridging the histologic tumor-stroma ratio with biomarkers for systemic response, as reported by the blood-based Stroma Liquid Biopsy™ panel.”
To Learn More About Tumour-Stroma Ratio, visit:
http://www.watchstroma.com
To Learn More About Stroma Liquid Biopsy™, download the most recent whitepaper at:
https://www.biotechsupportgroup.com/v/vspfiles/templates/257/pdf/StromaLiquidBiopsyWhitepaper02162022.pdf
To Learn More About AlbuVoid™ and other Albumin & IgG Removal products, visit:
https://www.biotechsupportgroup.com/Albumin-Removal-s/307.htm