The Utility of HemoVoid™ is Demonstrated in 3 Proteomic Investigations Identifying Potential Disease Specific Biomarkers
Background
Red Blood Cells (RBCs) are the subjects of extensive research, due to their functional relevance within associated disease states, such as sickle cell anemia, Paroxysmal Nocturnal Hemoglobinuria, thalassemia, malaria, and Parkinson’s Disease, to name a few. While the supply of oxygen and removal of carbon dioxide is a major function of red cells, red cells nevertheless serve many other functions, including Complement and inflammation regulation. Comparative proteomic studies have yielded new clues to the processes that regulate red cell membrane–cytoplasm interactions in health and disease, and to the ways by which red blood cells communicate with their environment. In addition, proteomic and metabolomic data have revealed many unsuspected metabolic processes taking place in the red blood cell cytoplasm. Taken together, red cells are at the convergence of many cellular and extracellular events to which all blood-borne processes coalesce.
Lacking a nucleus, mature RBCs are unable to synthesize new proteins in response to outside stimuli, making RBCs important reservoirs for biomarker research. Any changes in the RBC proteome content or structure due to disease, should be discernible once a baseline of normal/healthy controls is established. While many proteomic efforts have focused on the red cell membrane, less attention has been paid to the cytoplasmic fraction, mostly due to the presence of the super-abundance of hemoglobin. This is no longer an impediment to biomarker research on red cell lysates as BSG products have now been demonstrated to efficiently remove hemoglobin from both reticulocyte and mature erythrocyte lysates, enabling such in-depth proteomic investigations to flourish.
Solution
As one of the key BSG Advantages, Enrichment/Depletion, BSG offers a choice of two strategies for proteome enrichment, to get the best results: either selectively binding high abundance proteins (the “Bind” products), or choosing not to bind (the “Void” products) thereby enriching the underlying low-abundance sub-proteome. From a synthetically derived bead library of weak affinity, or imperfect fit porous beads, progressive displacement at or near saturation, allows the beads to bias for or against certain proteins. Specific trademarked products were empirically characterized to meet the needs of the application. HemoVoid™, is employed to remove hemoglobin from erythrocyte lysates in a simple and efficient manner, through a negative selection strategy, meaning the Hemoglobin voids out through the beads, and the low-abundance sub-proteome binds to the beads. For subsequent analysis, the bead-bound enriched proteome can either be eluted, or optionally for LC-MS, digested on-bead. This latter process is called Bead-assisted Sample Prep or BASP™. Outcomes
Three journal reports describe the utility of BSG’s Hemoglobin Removal products in the label-free LC-MS proteomic analysis of red cells and whole blood. 1) Das, Sonu, et al. "A journey to unravel the pathophysiology of stable and exacerbated Chronic obstructive pulmonary disease through erythrocyte proteomics: A combined mass spectrometry/bioinformatics approach." (2022). Chronic Obstructive Pulmonary Disease (COPD) is a progressive lung disorder with high mortality. This study, explores the novel and highly enriched protein networks differentially expressed in stable and exacerbated COPD variants to elucidate the disease pathophysiology. A label free relative quantification of erythrocyte cytosol proteome based on LC-MS/MS was performed on hemoglobin- depleted erythrocyte lysate samples of stable and exacerbated COPD, relative to healthy controls. To deplete Hemoglobin, the article states “HemoVoid™, a silica-based protein enrichment matrix from Biotech Support Group USA, was used to remove hemoglobin from erythrocyte lysate samples to unmask low abundance…proteins according to the manufacturer’s protocol.” 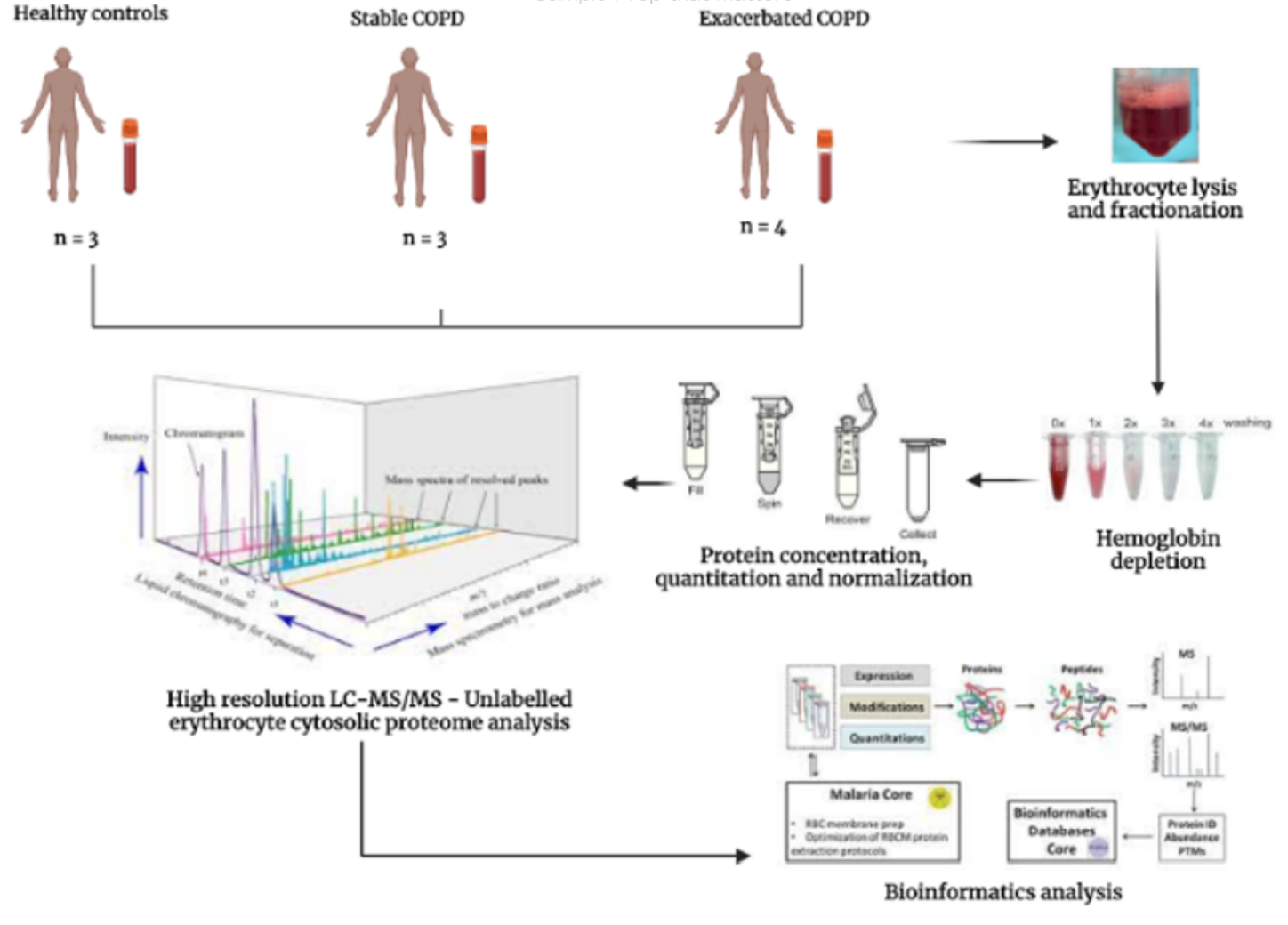
The article describes the observation of five highly enriched protein clusters in stable and seven in exacerbated COPD. Such differentially expressed proteins brought to light the dysregulation of molecular events such as ERAD pathway, MAPK signaling, ciliogenesis, hypoxia, apoptosis and neutrophil migration, resulting in the chronic inflammatory response characteristic of COPD. The hemoglobin depleted cytosolic proteins which are unique to exacerbated COPD, such as kyphoscoliosis peptidase, sperm associated antigen-1, LINE 1 and calpastatin could help in differentiating stable and exacerbated COPD clinically. 2) Wu, Na, et al. "Proteomic characteristics of plasma and blood cells in natural aging rhesus monkeys." Proteomics: 2200049. The underlying mechanisms of aging remain unclear. This study sought to understand the aging process as it may be affected by proteins in the blood, the most important functional system for material transportation in the body. For this purpose, the investigation analyzed and compared the protein expression spectrums in the blood of old and young rhesus monkeys. To extract blood cell proteins and deplete Hemoglobin, the article states “Blood cell proteins were lysed by incubation with protein extraction solution (Bestbio, China)…for 30 minutes…After centrifugation…the supernatants were further depleted of Hemoglobin using HemoVoid™ (Biotech Support Group) as per the instructions of the manufacturer.” Upon depletion, the study found 1183 proteins expressed differentially in blood cells. The study confirmed that the protein levels of complement C2 in plasma and actin-related protein 2/3 complex subunit 2 (ARPC2) in blood cells decreased, whereas the complement C3 and complement component 4 binding protein beta (C4BPB) significantly increased in plasma of old rhesus monkeys and C57BL/6 mice. The results suggest that C2, C3, C4BPB, and ARPC2 can be used as target proteins for anti-aging research. 3) Liu, Wenli, et al. "Erythroid lineage Jak2 V617F expression promotes atherosclerosis through erythrophagocytosis and macrophage ferroptosis." The Journal of Clinical Investigation (2022). 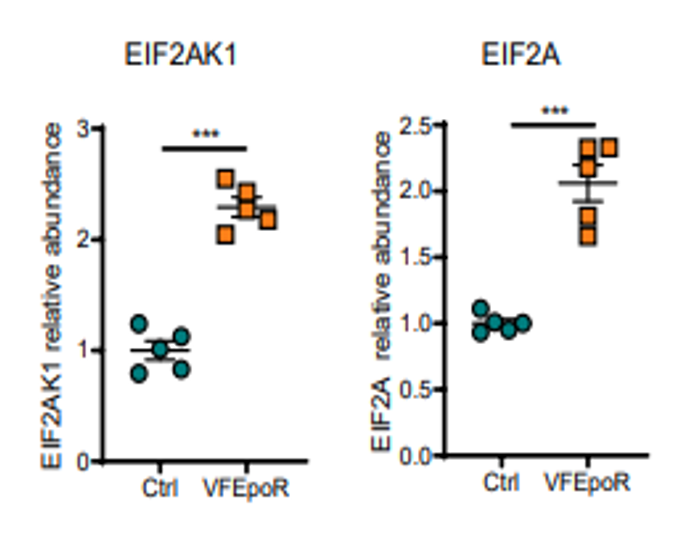
The JAK2V617F (Jak2VF) mutation increases cardiovascular risk in myeloproliferative disorders and in clonal hematopoiesis. To investigate whether selective erythroid Jak2VF expression promotes atherosclerosis, the investigators developed hyperlipidemic Erythropoietin Receptor Cre mice that express Jak2VF in the erythroid lineage (VFEpoR mice). To explore underlying defects promoting oxidative changes in Jak2VF Red Blood Cells (RBC), unbiased proteomics profiling was conducted. The article states “…haemoglobin removal and on-bead digestion, …was based on the protocol in HemoVoid kit. … Samples were first digested with LysC in HVWB (from kit) with protease: protein ratio 1:100 overnight at 37 °C and then with trypsin in HVWB (from kit) with protease: protein ratio 1:30.” The proteomic data showed a prominent increase in EIF2AK1 (also known as heme-regulated inhibitor, HRI) and EIF2A (above), consistent with increased oxidative stress. Also, there were decreased levels of three key enzymes in glutathione metabolism in VFEpoR RBC. The authors conclude that Erythroid lineage Jak2VF expression leads to qualitative and quantitative defects in RBCs that exacerbate atherosclerosis. These three references illustrate how BSG’s products and methods can efficiently remove Hemoglobin, enrich the low-abundance sub-proteome, and subsequently enable proteomic analyses to identify potential disease specific biomarkers from whole blood and sedimented erythrocytes. Additional Key Advantages of HemoVoid™ - Consumable, cost-effective
- Hemoglobin voids in flow-through >98%, with <30 minute bind/wash/elute protocol
- Suitable for high-throughput quantitative analysis
- Sample types include red blood cells, whole blood, and dried blood cards
- Species agnostic, validated on human, mouse, sheep bovine, monkey
- Eluted proteins maintain their tertiary structure and functional characteristics and can be profiled by native PAGE, structural activity probes, or enzymatic assay
Optional on-bead digestion supplied as HemoVoid™ LC-MS On-Bead For more information on HemoVoid™, visit: http://www.biotechsupportgroup.com/HemoVoid-Hemogl... For more information of all of our Hemoglobin removal products, visit: https://www.biotechsupportgroup.com/Hemoglobin-Rem...
|