
Investigate out of the Venn Diagram box
NRicher™ Knowledgebase for Targeted Proteomic Enrichment
BACKGROUND
Blood is the body’s vehicle for the accumulative evidence of pathological insults for diseases. Soluble plasma proteins, extracellular vesicles, and circulating blood cells mediate individualized homeostasis via intercellular communication, immune responses, vascular and endothelial cell function, tissue remodeling, fluid exchange, and nutrient assimilation. Thus, most diseases are multi-factorial with many proteins collectively acting within highly regulated networks – even single gene diseases can be at the center of larger, complex networks. Plasma/serum proteins and other circulating factors directly regulate complex processes such as aging, the development of chronic diseases (cancer, cardiovascular, neurodegenerative, etc.), and severe acute disease (i.e., sepsis, acute respiratory distress syndrome). A disease state results whenever this finely tuned protein system becomes dysregulated via a confluence of age, infectious pathogens, heredity, lifestyle, or environmental stimuli.
CHALLENGE
Blood mediates coordination between nonadjacent tissues, so it is essential to understand how dysregulation manifests itself, regardless of the underlying causative factors.
Quantitative proteomics from blood can help unravel these regulatory elements. Yet, extracting and characterizing functional changes and adaptations to disease for many of even the highest abundance proteins in circulation remains limited.
An over-emphasis on proteome coverage, across different platforms, has presented many Venn Diagram comparisons of analytical platforms and methods, but reduction to practical and actionable biomarker quantitative differentiation remains elusive.
Cost-effective and scalable targeted proteomic workflows is challenging.
The preponderance of high abundance proteins complicates efficiency and consistency in quantifying target peptides from different sample cohorts in targeted proteomic workflows. This is in part due to the changing landscape of proteins/peptides not associated with the selected targets, oftentimes called analytical matrix effects, or background noise.
SOLUTION
The NRicher™ Knowledgebase & Supporting Products/Methods
Purpose of the Knowledgebase
Researchers can determine whether pre-identified protein targets of interest can be enriched by one or more NRicher™ beads; user defined targets may come from sources such as:
-
Discovery proteomics
- Gene Expression
-
Curated from public domain databases and publications
Function of the Knowledgebase
Over 2000 proteins observed with corresponding relative signal intensities
Find your protein(s) of interest, and corresponding bead/methods to best enrich for protein(s) of interest, free to review, accessible on a non-confidential basis
Annotation of 200 soluble membrane proteins, derived from ectodomain shedding, a common dysregulated disease mechanism, and important source of precision biomarkers
Annotation of 100 prospective biofluid biomarkers associated with disease conditions
New strategies to compartmentalize and profile systemic chronic inflammation, and associated diseases
Why Choose NRicher™
For over 10 years BSG has been at the forefront of developing synthetic beads (i.e., ionic, hydrophobic, hydrogen bonding, aromatic, polymeric) with differential proteome binding properties.
The NRicher™ Advantage
Not derived from immuno-affinity: NRicher™ beads, are not species-specific. This allows a wider applicability across various sample types.
Cost-efficient:
There's no need for an investment in high-end specialized equipment; a standard laboratory microfuge will suffice.
Streamlined Analysis:
Through the use of bead cocktails, NRicher™ products serve virtually all applications, starting with sample volumes as low as 25 µl.
On-bead digestion:
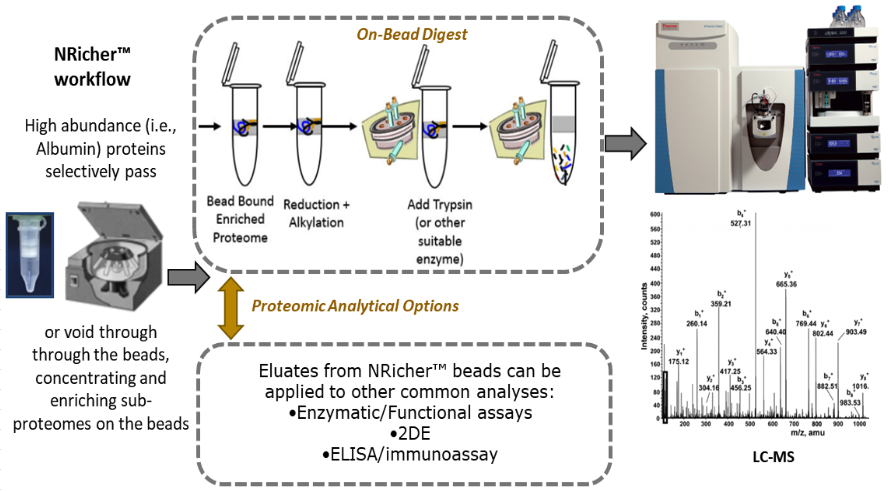
BSG pioneered Bead-Assisted Sample Prep (BASP™), offering workflow efficiencies (i.e., no added denaturants) for LC-MS proteomics of bead enriched sub-proteomes. The NRicher™, product line continues to advance sub-proteome enrichment, with products tailored to specific applications.
BSG’s fast and efficient sample prep products and methods allow quantitative monitoring of one or more blood proteins using simple microfuge workflows, no expensive instruments required.
Targeted protein enrichment improves signal to noise, as co-eluting peptides from non-targets can suppress ion-signal and interfere with spectral identifier assignments. BSG’s products enrich potential protein biomarkers of low-abundance to MS signals typical of mid to high abundance, and usually 5-10X relative to Albumin.
Low signals are caused by many reasons, the most obvious being protein level low abundance. However, there are other reasons for low signal, such as when pooled samples are used in TMT labeling, or when specific peptide features like amino acid variants or PTMs, may only occur infrequently and become hard to observe. So, with NRicher™ protein level enrichment, peptide injection loads can be much higher than without enrichment, achieving better signal to noise, especially at lower end signals.
Choose Best NRicher™ Enrichment Products for Your Biomarkers of Interest. Here’s How.
The Knowledgebase is pr
Columns C-P can be decoded to the bead(s)/method by contacting us.

Relative signal levels can be evaluated by ratios of target signal to either total signal or high abundance (i.e., Albumin) signal, or co-eluting peptide signal(s) associated with spectral interferences.
Other Tabs provide additional context to the Knowledgebase, such as the “Biomarker” Tab, here a few examples from that Tab:

Other Annotated tabs Include:
oluble Membrane Proteins.
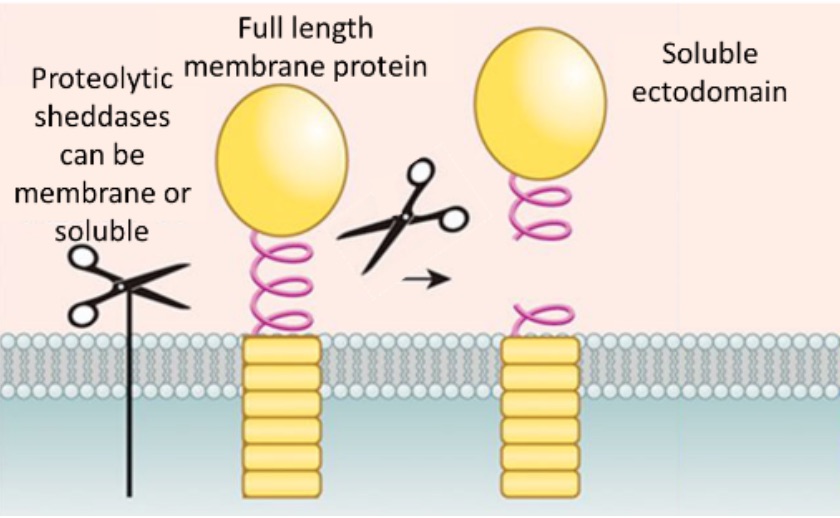
Through a regulated proteolytic mechanism called ectodomain shedding, the extracellular domains of membrane-anchored proteins are sometimes released from the cell surface as soluble proteins. During the shedding process, a protease (referred to as sheddase) cleaves a membrane protein substrate close to or within its transmembrane (TM) domain, resulting in release of the soluble extracellular domain (ectodomain) from the membrane and a fragment that remains bound to the membrane (Figure). Cells use ectodomain shedding to regulate the expression and function of surface molecules, and modulate a wide variety of cellular and physiological processes, including growth factor signaling, cell adhesion, inflammation and proliferation. As a result, any dysregulation within shedding processes can result in diverse pathologies such as inflammatory lung injury, infection, cancer, and cardiovascular disease.Therefore, proteomic profiling of the soluble ectodomain sub-proteome provides opportunities to discover important drug targets or personalized biomarkers. There are 200 proteins listed in the Knowledgebase as soluble membrane proteins from normal healthy sera. Given the exquisite enrichment bias towards the soluble membrane proteome, different disease sources of sera may provide additional soluble membrane protein observations, through the use of one or more NRicher™ products
Use the Knowledgebase for New Functional Proteomic Strategies To Profile Innate Immunity, Systemic Chronic Inflammation and Associated Diseases Chronic inflammation is strongly linked to the triangle of interconnected pathways of coagulation, complement and neutrophil recruitment. Indeed, the amplitude, and temporal dynamics of proteases and their inhibitors are critical factors influencing disease. Yet, conventional proteomic investigations on innate immunity proteins can often mislead, as common methods only produce static measurements, and assume a direct correlation of abundance to function. However, as the innate response is actived through proteolytic mechanisms, strict static measurements can be egregiously unreliable. The Knowledgebase offers strategies to help characterize these functionalities.
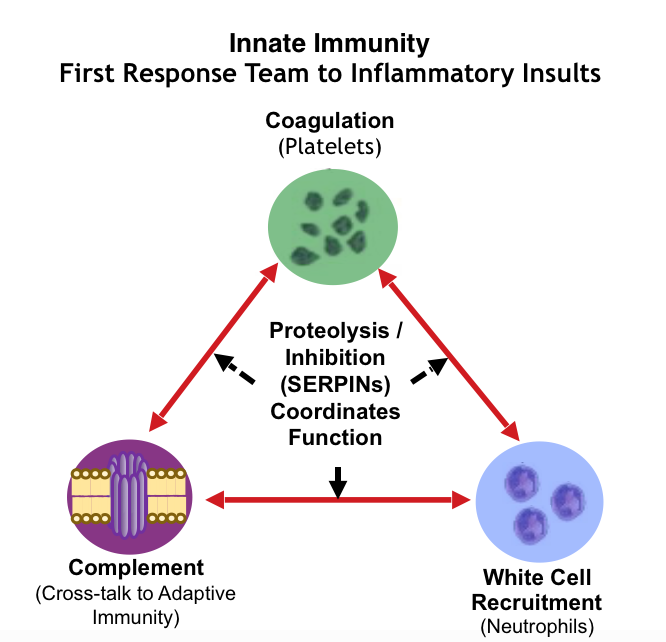
Proteomic biomarker profiles can characterize immune dysregulation with applications in early disease detection, risk evaluation, prognosis, and patient stratification. Many biomarker proteins listed mirror responses associated with acute phase inflammation, innate immunity (i.e., Complement), coagulation and degradation of platelets, extracellular matrix organization (soluble membrane proteins), as well as neutrophil heterogeneity/polarization and related granulocytic cargo release.
In addition to the “Biomarker” and “Membrane” Protein Tabs, we have assigned Tabs to categorical proteins according to:
-
Complement Activation and Regulation
- Coagulation Proteins and Platelet proteins, categorized by dense and alpha granules; granule secretion being pivotal to establishing and controlling the microenvironment at the local inflammatory site
- Neutrophils release cargo proteins from 5 different granule types in coordinated response to inflammatory insults; these are categorized
- Serpins: a very unique protein family of protease inhibitors, sometimes called suicidal inhibitors displaying a decoy trapping mechanism. Nine major inhibitory Serpins (i.e., Alpha-1-Antitrypsin, Antithrombin III) account for 5-10% of the protein mass in serum, affirming their importance to maintain normal homeostasis, and coordinating central control for innate immunity. Ask about new strategies to functionally monitor this essential protein family.
Conclusions
The open access is a valuable resource to find the best NRicher™ bead/method combination for your particular biomarker(s) of interest. A selection of one or more NRicher™ beads can be customized to meet the application requirements. Beyond the selection of bead chemistries, further optimization can be achieved through load and bind/wash buffer adjustments.
After NRicher™, target peptides have enriched spectral signal, even as gradient times are reduced NRicher™ sub-proteome enrichment can minimize acquisition time, collectively improving overall throughput, cost, and outstanding gains in productivity
Specific target peptides that report functional and gene variant regions promise actionable insights and potential multiplex biomarkers for disease.
NRicher™ methods offer scalability, automation compatibility, and cost effectiveness.
Click to Download the Most Current Knowledgebase.
For all inquiries about decoding the products and methods, please contact sales@biotechsupportgroup.com
|
|
|