Introducing NRicher™: Proteome Enrichment Simplified & Diversified
Biotech Support Group has achieved key milestones in proteomic research, pioneering innovative technologies in sub-proteome fractionation, functional proteomics, on-bead digestion, and targeted biomarker quantitation.
It is all based on the NRicher™ Bead Chemistry Platform, that started in 2010. The company goals were to apply new proteome subfractionation technology, not based on immuno-affinity, thereby generating sub-proteomes with reduced complexity and maintenance of functionality. Furthermore, we aimed to create consumable products with simple, cost-effective workflows, without the need for HPLC or additional capital expenditures.
Here is a quick overview of our innovations timeline and how the timeline to new innovations continues:
2010 Pioneering Bead-Based Proteomic Fractionations
The Company was the first to develop synthetically derived bead-based separations, not based on immuno-affinity, to differentiate sub-proteomes using simple bind-wash-elute methods
2012 First to Demonstrate Functional Proteome Analysis Using Bead-Based Separations
In this proof-of-principal paper, we examined bead-based differentiated tissue proteomes using functional proteomics analysis based purely on enzymatic reactions and substrate selection; a paper later cited in nanoparticle corona patent applications
1.
2015 Nature Publication & Industry Influence
In a 2015 Nature publication, third party researchers highlighted the NRicher™ beads (previously known as PROspector) as an enhanced enrichment step in proteomics workflows
2. The study demonstrated that twice the number of observations and annotations became possible, leading to a market shift away from antibody-based depletion products toward synthetic bead-based enrichment.
2015 Innovating On-Bead Digestion
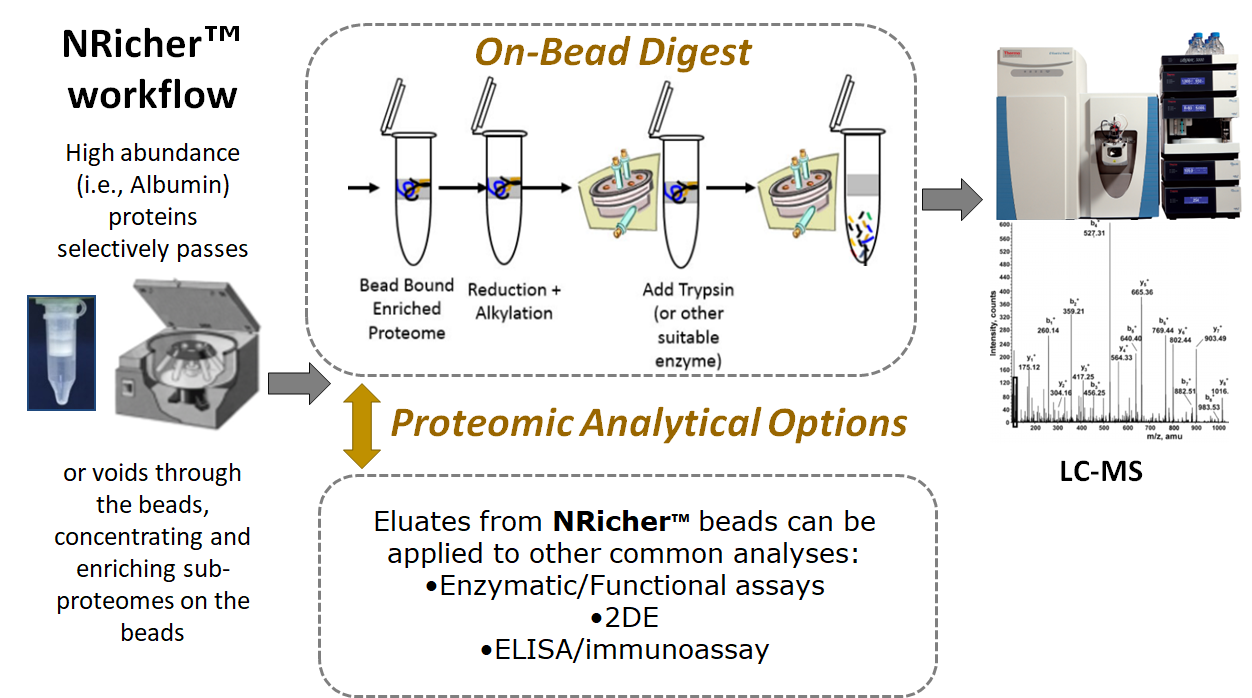
BSG pioneered on-bead digestion for albumin-depleted serum proteomes, significantly improving workflow efficiency and protein annotation compared to traditional depletion and in-solution digestion methods
3.
2016+ Commercial Success of Products for Albumin & Hemoglobin Removal
NRicher™-based platform has derived commercial products including
AlbuVoid™, HemoVoid™ and related fit-for purpose kits. Over 30 journal articles using these products have been published.
2024 Introduced NRicher™ Products for Family Specific Serum/Plasma Proteins
NRicher™-based products for soluble membrane proteins (Mx), Complement Cascade (C), Apolipoproteins (Apo) and Immunoglobulins (Ig) introduced.
2025 Advancing Targeted Proteome Enrichment
The NRicher™ platform presents an opportunity to enhance efficiency and productivity in proteomic workflows through precise sub-proteome enrichment. Researchers can leverage specialized bead chemistries tailored to specific applications, while further process refinements—such as buffer optimization—will streamline workflows, reduce acquisition time, and improve cost-effectiveness. Our open access NRicher™ Knowledgebase supports this opportunity. To accelerate commercialization, the Company secured a $40,000 NJEDA CSIT Catalyst II Research Grant, starting Q2 2025, to further accelerate NRicher™ bead chemistry characterization.
Each NRicher™ bead chemistry enriches low abundance biomarker proteins at remarkably different LC-MS signals (semi-quantitative), ideal for targeted and multiplex proteomics – some representative examples

2025+ Breakthroughs in Functional Proteoform and Inflammatory Differentiation
The Company is addressing a key challenge in proteomics: the inability of current ‘omic’ platforms to distinguish functional proteoforms and pathway outcomes. There remains a conventional proteomic assumption that all protein functions correlate to relative abundances, a carry-over viewpoint from gene expression. However, this assumption can be egregiously misleading regarding functional outcomes
4-6. The NRicher™ technology is designed to encourage the investigation of differentiated functional post-translational modifications (PTMs), and non-canonical variant regions, as well as imbalances due to activating and regulating sub-proteomes of innate immunity. Such innate imbalances are the drivers behind systemic chronic inflammation, a phenomenon propagating the single most medical burden in industrialized societies7,8. Consequently, there will be new avenues for biomarker discovery, and personalized medical decisions, overcoming the limitations of strictly counting proteins as a yardstick. We welcome inquiries to partner with us on our next wave of innovation.
The NRicher™ Advantage
For over 15 years BSG has been at the forefront of developing synthetic beads (i.e., ionic, hydrophobic, hydrogen bonding, aromatic, polymeric) with differential proteome binding properties. Many derivative products are well established in the field with numerous citations in leading journals. Following our lead to circumvent immuno-affinity, an approach combining corona-based separations adapted to magnetic nano-particles has been introduced. However, this necessitates a specialized instrument-based workflow, and the need to perform LC-MS analysis on multiple bead-derived sub-proteomes. Consequently,
NRicher™, offers a solution that stands out in its simplicity and versatility.