Cleanascite™ Unlocks Insights into Lipid-Driven Tumor Immunosuppression
Background
Lipid metabolism plays a central role in shaping the tumor microenvironment (TME). Beyond fueling cancer cell growth, tumor-derived lipids reprogram surrounding stromal and immune cells, often tipping the balance toward immune suppression and disease progression. Tumor-associated macrophages, neutrophils, and fibroblasts can all be influenced by abnormal lipid accumulation, which in turn promotes tumor growth, metastasis, and therapy resistance.
To decipher these mechanisms, researchers require tools that can selectively remove lipids without disrupting other cellular functions—allowing them to untangle the lipid-specific influences within complex cellular systems.
Challenge
In vitro cancer models—including 2D cultures, 3D spheroids, and co-culture systems—offer a platform to study the TME. However, pinpointing the exact contribution of lipids remains difficult.
Researchers require a method that can:
- Efficiently deplete lipids from tumor-derived supernatants and conditioned media.
- Preserve the integrity of non-lipid cellular signals and function.
- Be easily adapted across cancer models, including immune cell and fibroblast co-cultures.
Solution: Cleanascite™ Lipid Removal Reagent
Cleanascite™, an aqueous suspension-based reagent, enables
selective removal of lipids and lipoproteins from biological samples while maintaining the functional integrity of other cellular components. Unlike other lipid depletion methods, Cleanascite™ provides a physiologically compatible approach to study lipid-driven mechanisms in vitro and ex vivo. This allowed researchers to isolate lipid-driven effects, advancing our understanding of immunosuppressive signaling in cancer.
Case Examples
1. Neutrophil-Derived Immunosuppression
Study: Dahal, Ankit, et al. "Platelet-activating factor (PAF) promotes immunosuppressive neutrophil differentiation within tumors." Proceedings of the National Academy of Sciences 121.35 (2024): e2406748121.
Model: Murine cancer cell supernatants (KCKO, E0771) incubated with naïve neutrophils.
Key Insight: Lipid depletion with Cleanascite™ did not alter neutrophil activation but abrogated Arg1 and dcTrail-R1 expression, preventing the aquisition of Polymorphonuclear (PMN)- myeloid- derived suppressor cells (MDSC) phenotype.
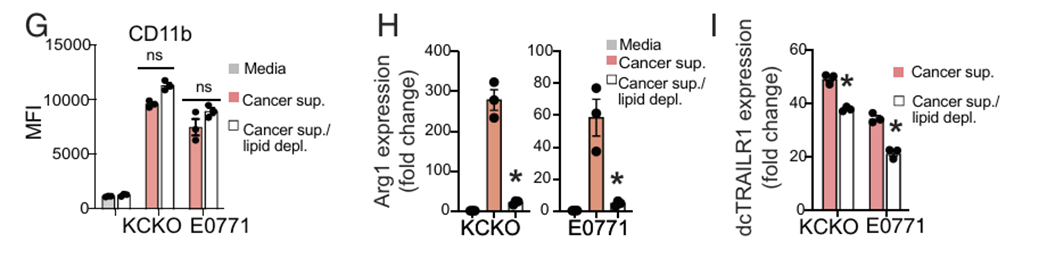
Conclusion: Cancer-secreted lipids are necessary mediators of neutrophil immunosuppressive reprogramming.
2. Environmental Carcinogen-Induced Cholesterol Reprogramming
Study: Zhang, T., Zhao, F., Hu, Y. et al. Environmental monobutyl phthalate exposure promotes liver cancer via reprogrammed cholesterol metabolism and activation of the IRE1α-XBP1s pathway. Oncogene (2024). https://doi.org/10.1038/s41388-024-03086-1
Model: HepG2 hepatocellular carcinoma cells exposed to monobutyl phthalate (MBP).
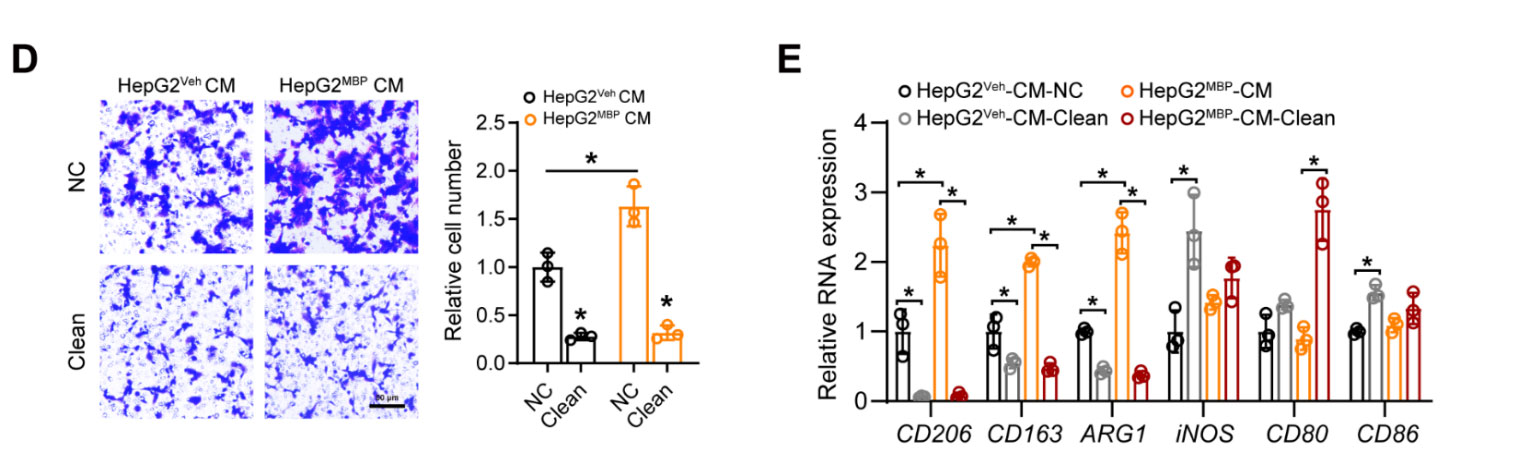
Key Insight: Cleanascite™ removal of lipids from conditioned media suppressed macrophage migration and M2 polarization (↓CD206, CD163, ARG1; ↑iNOS, CD80, CD86).
Conclusion: MBP exposure promotes tumorigenesis via lipid-dependent macrophage polarization that Cleanascite™ depletion effectively reversed.
3. Ovarian Cancer TAM Polarization via PUFAs
Study: Chan, David, et al. "Polyunsaturated Fatty Acids Promote Protumoral Macrophage Polarization via a RhoA-YAP1 Signaling Pathway in the Ovarian Cancer Microenvironment." (2022).
Model: Omental conditioned media and malignant ascites applied to macrophages.
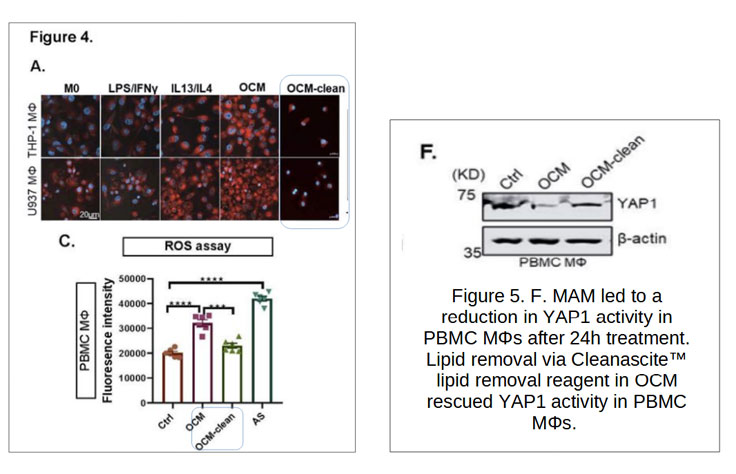
Key Insight: Lipid removal by Cleanascite™ reduced lipid droplet accumulation, mitigated ROS production, and restored YAP1 expression in macrophages.
Conclusion: Polyunsaturated fatty acids (PUFAs) in ascites are direct drivers of M2-like TAM polarization through the RhoA-YAP1 axis.
4. Oxidized LDL and TREM2+ TAMs in HCC
Study: Chu, T., Zhu, G., Tang, Z. et al. Metabolism archetype cancer cells induce protumor TREM2+ macrophages via oxLDL-mediated metabolic interplay in hepatocellular carcinoma. Nat Commun 16, 6770 (2025). https://doi.org/10.1038/s41467-025-62132-y
Model: Tumor interstitial fluid (TIF) from hepatocellular carcinoma (HCC) patients and mouse models.
Flow
cytometry of TREM2 and SPP1 expression in macrophages treated with TIF±Na2CO3
or lipid removal reagent (Cleanascite™).
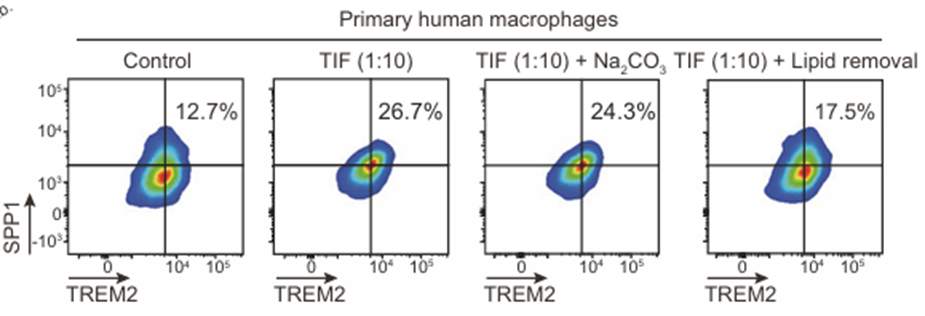
Key Insight: Cleanascite™ lipid depletion abolished the induction of TREM2 and SPP1 in macrophages by TIF, confirming that oxLDL mediates TAM polarization through the TREM2–SYK–CEBPα pathway.
Conclusion: Lipid cues from HCC cells directly enforce immunosuppressive macrophage re-programming and therapy resistance.
5. Fibroblast-Mediated Glutamine Synthesis
Study: Xiaoyun Li, Sofie Hedlund Møller, Jaeoh Park, Yu-Ming Chuang, Pei-Chun Hsueh, Tzu-Hsuan Chang, Kung-Chi Kao, Hector Gallart-Ayala, Yi-Hao Wang, Jhan-Jie Peng, Alessio Bevilacqua, Yi-Ru Yu, Zhiyu Li, Yann Kieffer, Domitille Peigney, Hugo Croizer, Yingxi Xu, Alfred Zippelius, Isabel C. Lopez-Mejia, Lluis Fajas, Fatima Mechta-Grigoriou, Julijana Ivanisevic, Zhengtao Xiao, Ming-Chih Ho, Ying-Chun Shen, Ping-Chih Ho; Tumor-instructed glutamine synthesis in cancer-associated fibroblasts promotes pro-tumor macrophages. J Exp Med 1 September 2025; 222 (9): e20241426. doi: https://doi.org/10.1084/jem.20241426
Model: Tumor-conditioned media (TCM) applied to cancer-associated fibroblasts (CAFs).
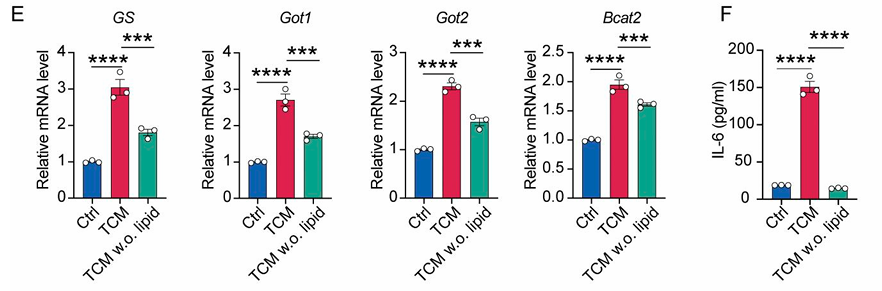
Key Insight: Lipid-depleted TCM significantly downregulated IL-6 and glutamine synthesis pathway genes in fibroblasts, impairing their ability to promote TAM polarization.
Conclusion: Tumor-derived lipids shape fibroblast metabolism, driving inflammatory CAF (iCAF) formation and sustaining macrophage immunosuppression.
Impact
By enabling precise lipid-selective depletion, Cleanascite™ has become an indispensable reagent for cancer immunometabolism research. Its use across multiple studies illustrates how dissecting lipid-driven signaling within the TME can reveal novel pathways of tumor progression and immune evasion.
Why It Matters
Lipid metabolism is emerging as a central node in cancer biology. Sample prep tools like Cleanascite™ allow researchers to:
- Unravel lipid-specific contributions to tumor-immune crosstalk.
- Identify therapeutic targets that disrupt lipid-driven immune suppression.
- Strengthen preclinical cancer models by eliminating confounding variables.
As more studies demonstrate lipid metabolism’s role in cancer progression, Cleanascite™ will remain essential for advancing discoveries that may ultimately improve patient outcomes.
|