 |
Cleanascite LX
For Lipemic Serum/Plasma Clarification
- Effectively replaces chlorinated/fluorinated hydrocarbons (eg. freon)
- Based on solid-phase referenced in over 70 publications in varied applications
- Under investigation as alternative to LipoClear for lipemic serum/plasma
Protocol
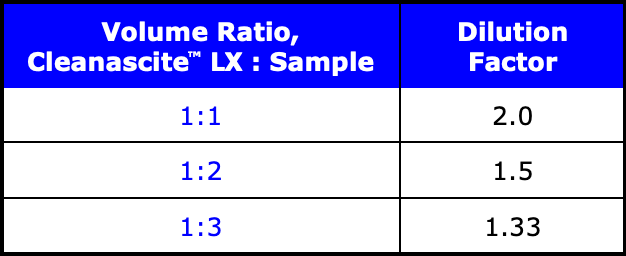
Cleanascite™ LX is supplied as an aqueous suspension of non-ionic adsorbent in DI water, pH 8.0. After centrifugation, the pellet is 1/2 of the total volume and the supernatant is 1/2 of the total volume.
Click Here To View Cleanascite™ LX Product Sheet
- Resuspend Cleanascite™ LX by gentle shaking. Excessive shaking may cause foaming. It should be completely resuspended prior to use.
- Add Cleanascite™ LX to the sample at minimum 1:3 (or alternative higher) ratio. Mix the sample by gentle shaking for 20 minutes.
- Micro-centrifuge sample at 8,000 rpm’s (5,000xg) for 10 minutes.
- Carefully aspirate supernatant for analysis.
Optimization. Different sample volumes are easily scaled. Volume ratio can be adjusted up or down as required to remove the amount of impurities present.
Cancer cell-conditioned medium Wang, Haiping, et al. "CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors." Nature Immunology (2020): 1-11.
Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their lipid clearance sample preparation technology for studying cell response factors associated with intratumoral Treg cells. Depleting regulatory T cells (Treg cells) to counteract immunosuppressive features of the tumor microenvironment (TME) is an attractive strategy for cancer treatment. However, systemic impairment of their suppressive function limits its therapeutic potential. Elucidating approaches that specifically disrupt intratumoral Treg cells is direly needed for cancer immunotherapy. The use of Cleanascite™ helped demonstrate that intratumoral Treg cells increase lipid metabolism and CD36 expression. The article states “cancer cell-conditioned medium … was treated with Cleanascite reagent (Biotech Support Group) before Treg cell culture at a volume ratio of 1:5 according to the manufacturer’s instructions.” The study concludes that CD36 targeting elicited additive antitumor responses with anti-programmed cell death protein 1 therapy. The findings uncover the unexplored metabolic adaptation that orchestrates the survival and functions of intratumoral Treg cells, and the therapeutic potential of targeting this pathway for reprogramming the tumor microenvironment. “Its rewarding to see another reference whereby Cleanascite™ helped to identify a characteristic feature of the tumor microenvironment. As Cleanascite™ is an aqueous suspension product without harmful solvents, one can use it to challenge cells for responsiveness. This ultimately helps researchers decide whether or not lipids impart characteristic changes in cells, such as the relative expression of membrane receptors like CD36. This information will hopefully help our understanding of how to reprogram the tumor microenvironment for therapeutic benefit.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Omental conditioned medium (OCM)
Chen, Rain R., et al. "Targeting of lipid metabolism with a metabolic inhibitor cocktail eradicates peritoneal metastases in ovarian cancer cells." Communications Biology 2 (2019).
Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their lipid clearance sample preparation technology for studying cell response associated with peritoneal metastases in ovarian cancer cells. Ovarian cancer is an intra-abdominal tumor in which the presence of ascites facilitates metastatic dissemination, and is associated with poor prognosis. However, the significance of metabolic alterations in ovarian cancer cells in the ascites microenvironment remains unclear. In this study, the authors investigated whether reprogramming of lipid metabolism in ovarian cancer cells could modulate cell viability and aggressiveness. The article states: ”To determine whether fatty acids in OCM are the primary energy source, fatty acids from OCM was first removed by Cleanascite™ Lipid Removal Reagent... Then, XTT cell proliferation assays showed that the growth rate of ovarian cancer cells was remarkably reduced in cells cultured in Cleanascite-treated OCM. Likewise, co-treatment with Cleanascite and OCM significantly attenuated the increased cell migration and invasion capacities of ES-2 and SKOV3 cells. These findings suggest that the fatty acid-enriched OCM provides as an energy source for supporting tumor growth and aggressiveness of ovarian cancer cells.”. The authors conclude that targeting the lipid metabolism signaling axis impedes ovarian cancer peritoneal metastases. “I am pleased that using Cleanascite™ helped to identify fatty acids as a mediator of cancer cell response. This demonstrates the advantage of using an aqueous suspension product with no harmful solvents, so one can challenge cells for responsiveness. This ultimately helps researchers decide whether a lipid factor is or is not consistent with cancer growth and metastatic potential. We highlight many such cell response references in our recently published whitepaper.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Astrocytes
Nguyen, Amanda. "HDLs and LDLs in the Brain: Characterizing the Lipid Secretome of Astrocytes." The FASEB Journal 31.1_supplement (2017): 948-3.
Astrocytes are glial cells of the nervous system. Astrocytes help to synthesize and transport lipids. Astrocytes produce particles composed of HDL/LDL-like particles which function in maintaining the lipid membrane. Astrocytes synthesize apolipoprotein E (apoE) which helps in transporting cholesterol to neurons. Maintaining proper neuronal function requires regulation of lipids. Scientists isolated from the primary astrocytes the lipid secretome using Cleanascite™. Thin layer chromatography was used to observe lipids.
Human serum, mouse serum or DMEM
Lee,
Hong-Jai, et al.
"Regulatory
effect of humoral milieu on the viral DNA and surface antigen
expression of hepatitis B virus (HBV) in vitro."
Molecular
& Cellular Toxicology
15.2 (2019): 123-128.
Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their lipid clearance sample preparation technology for determining variability in the in vitro regulation of Hepatitis B virus (HBV). The investigations explored if humoral milieu such as serum or culture media, and its constituents, and pH would regulate the viral DNA and surface antigen expression of HBV in vitro. Furthermore, lipid removal analysis showed decreased level of HBV DNA and surface antigen expression in human and mouse serum. The article states “To evaluate the lipid exposure status within lipid bilayer, Cleanascite (Biotech Support Group) was added to HBV mixtures in the human serum, mouse serum, or DMEM, and the HBsAg and HBV DNA were evaluated. … we examined the virus-lipid interaction in non-host milieu, and compared the interaction between in host and non-host milieu. The levels of HBsAg and HBV DNA were significantly decreased with lipid removal by Cleanascite in mouse serum rather than human serum”. The authors’ concluded that humoral lipid might confer protection to virion against toxicants or hostile interaction with humoral components. “This article is another example for the value of Cleanascite™ as a serum delipidation reagent so as to understand the effects of lipids on the viability of viruses. It fits into many other applications that show its utility in generating in vitro cell response information.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Noncirculating Biological Fluids
Farina, Annarita. " Pre-fractionation of Noncirculating Biological Fluids to Improve Discovery of Clinically Relevant Protein Biomarkers." Proteomics for Biomarker Discovery. Humana Press, New York, NY, 2019. 23-37. Biotech Support Group reports on a recent book chapter describing the simplicity and efficiency of their lipid clearance sample preparation technology for reducing analytical variables in the proteomic comparison of body fluids. For proteomic biomarker discovery, it is necessary to bridge the gap between basic and applied research by complying with clinical requirements. This chapter provides key suggestions for improving the discovery of clinically relevant protein biomarkers from body fluids. The chapter states : ”If the elimination of lipids…is necessary, the sample can by treated with lipid removal (Cleanascite)…“I am pleased that Cleanascite™ is cited as a general delipidation reagent for all the different body fluids that can be compared within a proteomic analysis for the discovery of new biomarkers.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Astrocyte conditioned media
Sprenkle, Neil T., et al. "Endoplasmic reticulum stress is transmissible in vitro between cells of the central nervous system." Journal of neurochemistry 148.4 (2019): 516-530.
Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their lipid clearance sample preparation technology for studying cell response factors associated with cells of the central nervous system.Improper protein folding and trafficking are common pathological events in neurodegenerative diseases that result in the toxic accumulation of misfolded proteins within the lumen of the endoplasmic reticulum (ER). Yet the cell‐extrinsic role of sustained unfolded protein response activation under physiological and pathological states in the central nervous system (CNS) remains to be elucidated. The authors studied the characteristics of a mediator secreted by ER stressed astrocytes and neurons. To determine if the mediator had lipid characteristics, the article states “…100 μl of Cleanascite slurry was added to 1 ml of conditioned medium and incubated at RT with end-over-end mixing for 1 h followed by centrifugation.” The authors provided evidence that depletion of lipids from astrocyte conditioned media using Cleanascite abrogated transmission of ER stress. Such evidence helped the authors conclude that ER stressed astrocytes and neurons secrete a molecule(s) with lipid characteristics which regulates both inflammatory and ER stress responses in other astrocytes, neurons, and microglia in vitro. These findings provide insight into the cell-nonautonomous influence of ER stress on cells of the central nervous system. “I am pleased that using Cleanascite™ helped to identify the characteristic feature of an unknown mediator of cell response. This demonstrates the advantage of using an aqueous suspension product with no harmful solvents, so one can challenge cells for responsiveness. This ultimately helps researchers decide whether any factor is or is not consistent with a biomolecule having lipid characteristics. We highlight many references in this area on our recently published whitepaper.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Whole mouse serum
Dean, E. Danielle, et al. "Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation." Cell metabolism 25.6 (2017): 1362-1373.
Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their lipid clearance sample preparation technology for studying factors associated with interrupted glucagon signaling. Decreasing glucagon action lowers blood glucose and may be useful therapeutically for diabetes. However, interrupted glucagon signaling leads to α cell proliferation. In this article, the authors wanted to determine which factors affected α cell proliferation. The article states “For lipid removal, whole mouse serum was treated with Cleanascite™ reagent (Biotech Support Group, Monmouth Junction, NJ) prior to islet culture at a 1:1 ratio according to the vendor’s protocol. Lipid removal was validated by HPLC to remove 99% of all phopsholipids, cholesterols, and triglycerides….”. In testing whether lipids could stimulate α cell proliferation, we found that serum activity was retained after the removal of >99% of triglycerides, cholesterols, and phospholipids. The authors conclude that amino acids, especially L-glutamine, regulate α cell proliferation and mass via mTOR-dependent nutrient sensing. “This is another application whereby using Cleanascite™ demonstrates the advantage of using an aqueous suspension product with no harmful solvents, to deplete lipid associated factors that may influence cell response investigations.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Plasma
Swertfeger, Debi K., et al. "Feasibility of a Plasma Bioassay to Assess Oxidative Protection of Low Density Lipoproteins by High Density Lipoproteins." Journal of Clinical Lipidology (2018). HDL can function in cholesterol efflux and atheroprotection. Authors Swertfeger, Debi K., et al evaluate if HDL provides protection from LDL oxidation. LDL modification and oxidation caused by water soluble radicals and copper oxidation is measured by gel filtration chromatography.Scientists find albumin is a significant source of antioxidation in copper initiated mechanisms. Uric acid, ascorbate, fibrinogen and immunoglobulin G are major antioxidative contributors. However measurement of HDL's antioxidative function in plasma requires HDL separation from plasma.
Estrous sheep serum
Barrera, Natalibeth, Pedro C. dos Santos Neto, Federico Cuadro, Diego Bosolasco, Ana P. Mulet, Martina Crispo, and Alejo Menchaca. "Impact of delipidated estrous sheep serum supplementation on in vitro maturation, cryotolerance and endoplasmic reticulum stress gene expression of sheep oocytes." PloS one 13, no. 6 (2018): e0198742.
Culture media containing high intracellular lipid droplets can alter oocyte cryo tolerance and cryopreservation resistance. Optimization of lipid content is important for oocytes research. High lipids may cause cryo injuries in cumulus oocytes complexes (COC). High lipids can alter competence of oocytes and alter embryo development. Methods on oocyte collection, in vitro maturation (IVM) and In vitro fertilization (IVF), lipid droplet staining can be enhanced by lipid content reduction. Lipids such as triglycerides, total cholesterol, nonesterified fatty acids from estrous sheep serum. Lipid removal protocol with Cleanascite™ allows authors Barrera, Natalibeth, et al. to evaluate embryo development, cryotolerance after vitrification, expression of endoplasmic reticulum stress genes in experiments. High lipid content of oocytes and embryos in domestic animals is one of the well-known factors associated with poor cryosurvival. In this articles, the authors wanted to determine whether the use of delipidated estrous sheep serum during in vitro maturation (IVM) of ovine oocytes reduces the cytoplasmic lipid droplets content and improves embryo development and cryotolerance after vitrification. The article states “Lipid removal from serum was performed by using Cleanascite™ (Biotech Support Group, NJ, USA) according to the instructions provided by the manufacturer. Unlike other approaches, the protocol described herein for delipidation of estrous sheep serum was effective in decreasing levels of Triglycerides, total Cholesterol, and NEFAs. To our knowledge this is the first study to use the Cleanascite method to generate estrous sheep serum yielding significantly reduced lipid levels. Subsequent use of the partially delipidated serum as supplemented in IVM media resulted in effective reduction of oocyte lipid content. The advantage of this method over other traditional methods (i.e. chloroform) includes increased feasibility and reduced toxicity and biosafety concerns” Their results demonstrate that although supplementation of IVM medium with delipidated estrous sheep serum reduces the presence of cytoplasmic lipid droplets in oocytes after maturation, oocyte cryotolerance is not improved. “I am pleased to see another example of the versatility of Cleanascite™. This demonstrates the advantage of using an aqueous suspension product with no harmful solvents, to deplete lipid associated factors that may influence cell response investigations.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Extracellular vesicles (EVs) from bone marrow (BM)-derived mesenchymal stromal cells (BM-MSC)
Nguyen, Doan C., Holly C. Lewis, Chester Joyner, Vivien Warren, Haopeng Xiao, Haydn T. Kissick, Ronghu Wu, Jacques Galipeau, and F. Eun-Hyung Lee. "Extracellular vesicles from bone marrow-derived mesenchymal stromal cells support ex vivo survival of human antibody secreting cells." Journal of extracellular vesicles 7, no. 1 (2018): 1463778.
EV's from BM-MSC secretome were evaluated for increased human IgG antibody secreting cells (ASC) survival. The EV's are linked to short and long distance signaling. Non-irridated and irridated EV's are analyzed. Larger particles and high concentrations were generally seen in non-irridated EV's. The EV's are related to clathrin mediated caveolin mediated signaling, vesicular transport, recipient cell uptake, integrins, integrins linked kinases, immune cell proliferation, protein translation and endocytosis. To evaluate IgG ASC survival the authors report using delipidated methods on ASC populations. The article states "Freshly sort-purified blood ASC populations were cultured in the irradiated (and non-irradiated) BM-MSC secretomes that was pretreated with Cleanascite (CLN), a lipid removal reagent (Biotech Support Group), according to the manufacturer’s recommendations. Similar experiments were also conducted using EV fractions derived from irradiated and non-irradiated secretomes. Secretomes and conventional media (R10) or PBS were used as controls."
MSC isolation and culture, culture medium preparation, EV purification, electron microscopy, cell isolation, FACS, peptide purification, IgG elispot assay, western blot, bioinformatics and statistical analysis is subsequently performed. The author report mRNA and microRNA in MSC derived EV's to research EV's and IgG ASC survival.
Pancreatic Rough Endoplasmic Reticulum (RER) Fractions
Waldron, Richard T., et al. "Ethanol Induced Disordering of Pancreatic Acinar Cell Endoplasmic Reticulum: an ER Stress/Defective Unfolded Protein Response Model." Cellular and Molecular Gastroenterology and Hepatology(2018).
The authors’ investigated the association of heavy alcohol consumption with pancreas damage, with consideration of light drinking that shows the opposite effects, reinforcing proteostasis through the unfolded protein response orchestrated by X-box binding protein 1. The article states that the endoplasmic reticulum “RER fractions (100 mg) were extracted by shaking for 15 minutes at 37°C in buffer comprising 100 mmol/L Tris pH 7.0, containing 25% acetonitrile, 0.1% sodium dodecyl sulfate, and 0.05% sodium deoxycholate (Buffer B), in 1.5 mL Eppendorf Protein LoBind tubes flushed of air using N2 gas. Next, 75 mL of a 1:1 slurry of Cleanascite delipidizing beads were added, and the tubes were flushed with N2 gas and rotated end-over-end for 15 minutes at room temperature. Slurries were spun at 10,000 rpm for 5 minutes at room temperature, clarified extracts were transferred to clean tubes, and pelleted beads were discarded.” The isolated ER proteome was then analyzed byOXICAT LC-MS. The study concludes that ethanol-induced changes in endoplasmic reticulum protein redox and structure/function emerge from an unfolded protein response–deficient genetic model. “This reference shows the versatility of Cleanascite™ to efficiently remove lipids from pancreas tissue extracts, so that cellular compartments can be investigated.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Human SZ95 sebocytes
Lovászi, M., et al. "Sebum lipids influence macrophage polarization and activation." British Journal of Dermatology (2017). doi: 10.1111/bjd.15754
Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their lipid removal sample preparation product for the differentiation and activation of macrophages based on a sebum lipid profile. The article’s authors report on sebum lipids contributing to the differentiation, polarization and function of macrophages. In order to determine the role of specific lipids, lipid removal was investigated from supernatants of the immortalized human SZ95 sebocytes, as stated, “For lipid depletion of the supernatants Cleanascite lipid clarification reagent (Biotech Support Group, Monmouth Junction, NJ, USA) was used according to the manufacturer’s instructions. Lipids; squalene, linoleic acid, oleic acid, palmitic acid and stearic acid (Sigma-Aldrich); were replaced individually subsequent to lipid depletion in a concentration of 150 μM.”. The authors concluded a role for sebaceous glands in modulating immune responses via their secreted lipids that are of possible pathologic and therapeutic relevance. “I am very pleased that this reference shows yet another sample type suitable for the selective efficiency of Cleanascite™, that is to remove sebum lipids for subsequent analysis using immunoassays.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Ascites Fluid
Chan, DW, Mak, SL, Ngan, HYS. The significance of lipid metabolism in peritoneal metastases of ovarian cancer. The 2016 Cold Spring Harbour Asia Conference on Cancer and Metabolism, Suzhou, China, 19-23 September 2016.
http://hub.hku.hk/handle/10722/235385
Biotech Support Group reports on a recent research poster describing the simplicity and efficiency of their sample preparation technology for clearing lipids from omental explant culture system, for the study of ovarian cancer cells and intraperitoneal tumor colonization. In brief, the authors report that the high lipid content in ascetic fluid provides a huge energy source for ovarian cancer cells in peritoneal dissemination and intraperitoneal tumor colonization. In this study, ovarian cancer cells co-cultured with an omental explant culture system (OCM) or ascetic fluid from ovarian cancer patients exhibited an increase in in vitro cell growth, cell migration/invasion through activation of TAK1/NF-kappaB signaling cascade. The abstract states “In contrast, the oncogenic capacities of ovarian cancer cells were impaired when cultured in OCM treated with Cleanascite Lipid Removal Reagent, suggesting that the bioactive lipids in OCM are required for enhanced oncogenic capacities”. “I am very pleased that the versatility of Cleanascite™ has helped to support a new area of research, studying the metabolic changes associated with cancer. The exquisite selectivity profile of Cleanascite™ makes this possible.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Featured Application - microRNA from Egg Yolk
Ben Wade, Michelle Cummins, Anthony Keyburn and Tamsyn M. Crowley. Isolation and detection of microRNA from the egg of chickens. BMC Research Notes 2016 9:283. DOI: 10.1186/s13104-016-2084-5
In
brief, the article’s authors report a method for the reproducible and reliable
isolation of miRNA from the albumen and yolk of chicken eggs. These methods
will allow the investigation of epigenetic programming in chick development
previously unknown, and how this impacts the nutritional value of eggs for
human consumption. The article states “
…400 µl
aliquots of the yolk/lysis solution was dispensed into five 1.5 ml microcentrifuge
tubes. To each of these aliquots 600 µl of Cleanascite™ was added followed
by rigorous vortexing until the sample became homogenous. The Cleanascite™
removes the lipid from this high fat tissue that would otherwise interfere with
the extraction process…"
“This is still another example of the versatility of Cleanascite™ as it now shown to help purify microRNA from egg yolk, a very lipid-rich tissue type. The exquisite selectivity profile of Cleanascite™ makes this possible.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Featured Application - Vaccine Research
Vaccine research relies on the systemic immunogenic response to the vaccine candidate. To evaluate such a response, it is necessary to measure the antibodies from sera; a sample type with a diverse lipid profile between individuals. Because lipids can often impact antibody analysis by specific and non-specific matrix effects, for vaccine development, it is beneficial to deplete lipids prior to analysis. The Biotech Support Group product – Cleanascite™ has the necessary selectivity profile to support this demanding application. Read more...
Serum or BloodBerggren, Per Olof, and Shao-Nian Yang. " Methods for treating and/or limiting development of diabetes." U.S. Patent Application No. 14/995,341. In diabetes patients report symptoms of
high glucose, proteinuria, insulin resistance, less insulin
production, pathological glomerular clearance in addition to
complications such as peripheral vascular disease, atherosclerosis,
smooth muscle cell dysfunction, stroke and atherosclerosis.
Researchers are developing test
compounds (such as antibody, aptamers, polypeptides, nucleic acids
etc) that can inhibit protein kinase A (PKA), protein kinase C(PKC)
and Src kinases. A new patent titled “Methods for treating and/or
limiting development of diabetes." with U.S. Patent Application
No. 14/995,341 describes the role of apolipoprotein CIII (apoCIII) in
diabetes pathology. Type 1 diabetes mellitus (T1DM) can contain T
lymphocyte mediated autoimmune attacks in addition to high entrance
of calcium to pancreatic B cells causing apoptosis. Whereas in type 2
diabetes(T2DM), there is less B cell mass, hyper-activation of B cell
calcium channels as shown in Goto Kakizaki rats. ApoCIII is on the
surface of high density lipoproteins (HDL), low density
lipoproteins(LDL), chylomicrons. It is made in the liver and
intestine. It is a diabetogenic serum factor linked to B cell
destruction, obesity, dyslipidemia. ApoCIII is involved in cell
signaling, binding to molecules (such as NF-kB, beta 1 integrins,
protein kinases, toll like receptors, surface receptors in scavenger
receptor B type 1) and in the inhibition of triglycerides. ApoCIII is
also linked to inflammatory processes and hyperactivation of voltage
gated calcium channels in pancreatic beta cells.In diabetes, voltage
gated channels can modulate insulin secretion, B cell development,
glucose metabolism, gene expression, protein phosphorylation, and
discovering candidate compounds to limit diabetes may require
researching apoCIII. For example, decreasing beta1 integrin activity
prevented apoCIII from hyperactivating B cell calcium channels.
Authors cite methods to detect apoCIII from serum or blood samples
with Biotech Support Group's
AlbuSorb™ to enhance subsequent
detection by MALDI-TOF mass spectrometry.
The patent provides descriptions of an
opportunity to design test compounds that are inhibitory towards the
activation of protein kinase A or Src kinase. The inhibitors can be
aptamers or antibody that bind to pancreatic B cells. In addition
designing integrin antisense oligonucleotides, beta1 cells integrin
shRNA, beta1 integrin siRNA, anti-beta1 integrin antibody, anti-beta1
integrin aptamers are ways to progress treatments of diabetes.
Finding the amount of apoCIII to increase density and conductivity of
calcium v1 channels and producing methods to limit diabetes with an
inhibitor of protein kinase A and Src kinase are drug discovery
pathways.
Plasma/Serum
Taylor, Steven W., Nigel J. Clarke, Zhaohui Chen, and Michael J. McPhaul. "A high-throughput mass spectrometry assay to simultaneously measure intact insulin and C-peptide." Clinica Chimica Acta 455 (2016): 202-208
Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their proteomic sample preparation technology for clearing lipid-associated matrix effects from human sera.
The citation is:Taylor, Steven W., Nigel J. Clarke, Zhaohui Chen, and Michael J. McPhaul. "A high-throughput mass spectrometry assay to simultaneously measure intact insulin and C-peptide." Clinica Chimica Acta 455 (2016): 202-208.
Available online 25 January 2016. doi:10.1016/j.cca.2016.01.019
In brief, the article’s authors aimed at simultaneously measuring intact insulin and proinsulin derived C-peptide, to help predict development of diabetes mellitus, as well as in differential diagnosis in cases of hypoglycemia. The article states “…15 µl of internal standard were added to each well followed by 50 µl of Cleanascite™ delipidation reagent previously mixed into a uniform suspension by a brief aspiration/dispense cycle within its reagent reservoir.” The article further notes a key component of the methodology as “…the use of a delipidation reagent to enhance immunocapture…The result was greatly enhanced recoveries and tighter CVs for the IS {internal standard} throughout the plate”.
“This is an exciting development as Cleanascite™ is shown both to improve LC-MS measurements, and validated in accordance with CLIA ’88 guidelines. Clearly, there was necessity for removing lipids without compromising the quantity or quality of the 2 biomarkers present and to be measured. The exquisite selectivity profile of Cleanascite™ makes this possible.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Knight, Jason S., Wei Luo, Alexander A. O'Dell, Srilakshmi Yalavarthi, Wenpu Zhao, Venkataraman Subramanian, Chiao Guo et al. "Peptidylarginine Deiminase Inhibition Reduces Vascular Damage and Modulates Innate Immune Responses in Murine Models of Atherosclerosis." Circulation research (2014): CIRCRESAHA-113.
Delipidation of serum using Cleanascite™ for peptidylarginine deiminase & atherosclerosis research. Neutrophil extracellular trap (NET) formation leads to thrombosis and blocking peptidylarginine deiminase (PAD) with Cl-amidine reduces atherosclerosis. NET formation is a marker for sepsis, cancer, thrombosis, autoimmune disease. Authors Knight et al published an article in the journal Clinical Research which cites Cleanascite™ from Biotech Support Group for lipid clarification and adsorption from serum samples. The article quotes, "Clearance of lipids from serum. Lipids were removed by Cleanascite Lipid Removal Reagent (Biotech Support Group, Monmouth Junction,NJ) according to manufacturer's instructions. The protocol removed >80% of total cholesterol and triglycerides."
Lijowski M, Caruthers S, Hu G.
High-Resolution SPECT-CT/MR Molecular Imaging of Angiogenesis in the Vx2 Model Investigative Radiology.2009;44(1): 15–22
For the preparation of nanoparticle molecular imaging agent that affords sensitive nuclear detection in conjunction with high-resolution MR characterization of tumor angiogenesis. Molecular imaging allows researchers to study integrins on proliferating endothelial cells during angiogenesis. Scientists combined 99mTc imaging and MRI to provide high sensitivity detection with high-resolution 3D neovasculature. Comixture of the integrin-targeted 99mTc nanoparticles included 3 mole% bis-pyridyl-lysine-caproyl-phosphatidylethanolamine, 0.1 mole% vitronectin antagonist complexed to PEG2000-phosphatidylethanolamine, and high purity egg phosphatidylcholine for balance. The surfactant comixture of the integrin-targeted particles 99mTc-gadolinium nanoparticles included 30 mole% gadolinium diethylene-triamine-pentaacetic acid-bis-oleate as an equimolar substitution for the lecithin. During the preparation of 99mTc-Tricarbonyl precursor and 99mTc nanoparticles Cleanascite™ HCl (300 μL) was added to the reaction mixture to precipitate the nanoparticles. The nanoparticles retained 97% of the 99mTc in plasma.
Turner JD, Langley RS, Johnston KL.
Wolbachia Lipoprotein Stimulates Innate and Adaptive Immunity through Toll-like Receptors 2 and 6 to Induce Disease Manifestations of Filariasis The Journal of Biological Chemistry.2009;284:22364-22378
In this article, researchers used Cleanascite™ first to determine if TLR2/6 ligands of Wolbachia are lipoproteins for removing lipids and lipoproteins. Next BindPro™, a polymeric protein removal suspension reagent (Biotech Support Group) was used to ablate levels of HEK-TLR2 cell IL-8 reporter gene activity to BMFE thereby showing that the TLR2/6 activity depends on both lipid and protein moieties.
Torrelles JB, DesJardin LE, MacNeil J. et al
Inactivation of Mycobacterium tuberculosis mannosyltransferase pimB reduces the cell wall lipoarabinomannan and lipomannan content and increases the rate of bacterial-induced human macrophage cell death Glycobiology.2009;19(7):743-755
Scientist isolated M.tb genomic DNA from cultures grown in 7H9 broth, OADC, 0.1% Tween 80. After centrifugation, the bacterial pellet was resuspended in water, packed volume sterile glass beads and 5 mL phenol: CHCl3 (pH 8). After vortexing and mixing the samples, the aqueous phase was removed following centrifugation Cleanascite™ from Biotech Support Group was used for removing lipids from samples. CHCl3:isoamyl alcohol (24:1),500 μL ProCipitate, and 3M sodium acetate was added to sample for DNA precipitation.
Castro AR, Morrill We, Pope V.
Lipid Removal from Human Serum Samples Clinical and diagnostic laboratory immunology.2000;7(2):197-199
Authors reviewed the efficacy of lipid removal containing antibodies to treponemal and nontreponemal syphilis antigens from human serum samples by using Cleanascite™ to the standard chloroform method. After administering Cleanascite™ or chloroform, the lipid content was measured before and after treatment. Amount of lipid removal ranged from 61 to 70% with Cleanascite™ and 60 to 62% with chloroform. Moreover, authors praise Cleanascite™ for being environmentally friendly than chloroform.
Cho N, Chueh PJ, Kim C et al
Monoclonal antibody to a cancer-specific and drug-responsive hydroquinone (NADH) oxidase from the sera of cancer patients. Cancer Immunology, Immunotherapy. 2002;51(3):121-9
Scientists prepared monoclonal antibodies to a 34-kDa circulating form of a drug-responsive hydroquinone (NADH) oxidase with a protein disulfide–thiol interchange activity specific to the surface of cancer cells and the sera of cancer patients. Cleanascite™ was used for deplipidation of sera. Epitopes (antibody (mAb) 12.1 and postimmune antisera ) inhibited the drug-responsive oxidation of NADH with the sera of cancer patients. Authors concluded both mouse ascites containing mAb 12.1 and postimmune sera (but not preimmune sera) slowed the growth of human cancer cell lines in culture, but did not affect the growth of non-cancerous cell lines.
Shapiro S, Beenhouwer DO, Feldmesser M et al.
Immunoglobulin G Monoclonal Antibodies to Cryptococcus neoformans Protect Mice Deficient in Complement Component C3 Infect. Infection and immunity.2002;70(5):2598-604
The effect of complement component C3 on mice affected by Cryptococcus neoformans was studied by researchers to determine the role of complement on Ab-mediated protection for four mice Ig subclasses (IgG1), IgG2a, IgG2b, IgG3 switch variants. Role of complement component C3 in Ab-mediated protection was determined by passive administration of MABs and reviewing the course of disease progression. Ascitic fluid was obtained by intraperitoneal (i.p.) injection of hybridoma cells into SCID mice. After centrifugation of ascetic fluid, Cleanascite™ protocol was implemented to remove lipids and cell debris. ELISA quantified the Ab concentration. Results showed IgG MAbs protect against cryptococcal infection in mice in the absence of C3.
Nussbaum G, Cleare W, Casadevall A et al
Epitope Location in the Cryptococcus neoformans Capsule Is a Determinant of Antibody Efficacy The Journal of experimental medicine.1997;185:685-694
For the preparation of monoclonal antibodies from ascites of hybridoma cells, Cleanascite™ was used for lipid removal. Monoclonal antibodies (mAbs) to the polysaccharide capsule of Cryptococcus neoformans can prolong survival in mice. epitope specificity in determining protective efficacy was suggested by experiments with two murine IgM anticryptococcal mAbs, 12A1 and 13F1. The protective mAb, 12A1, produced a homogeneous annular fluorescence pattern, whereas the nonprotective mAb, 13F1, produced a punctate pattern of fluorescence on one strain of serotype D, C. neoformans.
Palekar, Rohun U., et al. "
Thrombin-Targeted Liposomes Establish A Sustained Localized Anticlotting Barrier Against Acute Thrombosis." Molecular Pharmaceutics (2013).
Sample preparation via Cleanascite™ mediated delipidation of D-phenylalanyl-L-prolyl-L-arginyl-chloromethyl ketone (PPACK) for a localized site targeted nanoparticle-based antithrombotic agent utilized in the treatment of acute thrombosis.
Authors Palekar et al published an article in the journal Molecular Pharmaceutics citing Cleanascite™ lipid adsorption reagent used in protocols for delipidation of D-phenylalanyl-L-prolyl-L-arginyl-chloromethyl ketone (PPACK) coupled to liposomes in the supernatant from samples of human plasma containing clots. The article quotes Cleanascite™ "quantification of uncoupled PPACK recovered from the supernatant after centrifugation of pre-dialysis PPACK-Liposomes mixed with Cleanascite lipid adsorption reagent (Biotech Support Group, Monmouth, NJ). "Lipid conjugated PPACK increased localized anti-thrombin activity and could prolong the systemic clearance and the aqueous compartment could encapsulate drugs. Nano sized particles are researched as in vivo anticoagulants and as anti-coagulants.
McIntyre, John A., et al. "
Antiphospholipid autoantibodies as blood biomarkers for detection of early stage Alzheimer's disease." Autoimmunity0 (2015): 1-8.
R-RAA Antiphospholipid Antibodies (aPLs) Biomarker Discovery Research on Alzheimer's Disease (AD). Alzheimer's disease causes impairment in memory, language and learning. Genetic, cholinergic, amyloid, tau pathology of Alzheimer's disease requires discovery of biomarkers.R-RAA aPL is a biomarker for Alzheimer's disease (AD). R-RAA-aPLs are detected upon oxidizing agents being exposed to plasma, serum, cerbrospinal fluid (CSF) or immunoglobulin fractions. Research has shown R-RAA antiphospholipid antibody (APLs) are less in CSF and serum of AD than healthy controls. Authors McIntyre et al cite R-RAA-aPLs's in biomarker discovery research on mild cognitive impairment (MCI), AD and healthy controls. The experiment detected R-RAA aPL by ELISA. The R-RAA aPL in sera from AD diagnostic group were less than healthy controls, whereas the MCI group had increased R-RAA aPL activity. Larger samples may require proper sample preparation and such research is important to detect biomarkers on dementia of Alzheimer's type. Authors McIntyre et al cite Cleanascite™ from Biotech Support Group in the journal Autoimmunity. The citation quotes "Aliquots of the 90 ADNI serum samples were thawed and treated with Cleanascite™ (Biotech Support Group, Inc., North Brunswick, NJ) at a serum: Cleanascite™ ratio of 4:1 v/v in 2 ml microcentrifuge tubes with gentle rocking at 37 °C for 10 min".
Bile
Vesterhus, Mette, et al. " Novel serum and bile protein markers predict primary sclerosing cholangitis disease severity and prognosis." Journal of hepatology 66.6 (2017): 1214-1222. Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their lipid clearance sample preparation technology for the proteomic analysis of bile fluid. The investigators aimed to identify novel protein biomarkers of disease severity and prognosis in primary sclerosing cholangitis (PSC), using a bead-based array targeting 63 proteins. The article states for Bile sample preparation, “The samples were thawed and 100 µL of bile was added to 150 µL PBS containing 0.1% Tween 20 and Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, Missouri, USA), then centrifuged at 4 °C for 10 minutes at 14000 rpm. Cleanascite™ (100 µL, Biotech Support Group, NJ, USA) and Protein G Sepharose (50 µL; Sigma-Aldrich) were used for removal of lipids and immunoglobulins, respectively.” The authors conclude with the identification of novel biliary and serum biomarkers of severity and prognosis in PSC. “This reference shows the versatility of Cleanascite™ to efficiently remove lipids from bile fluid, with a selectivity profile suitable for proteome analysis using bead-based capture features.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group. Laohaviroj, Marut, et al. "A comparative proteomic analysis of bile for biomarkers of cholangiocarcinoma." Tumor Biology 39.6 (2017): 1010428317705764.
The article’s authors used a quantitative proteomics approach to identify potential tumor-associated proteins in the bile fluid of six cholangiocarcinoma patients. Cholangiocarcinoma is a primary malignant tumor of the bile duct epithelium and is usually detected at an advanced stage when successful treatment is no longer possible. As the tumor originates from the bile duct epithelium, bile is an ideal source of tumor biomarkers for cholangiocarcinoma. In this study, Isobaric labeling, coupled with Tandem mass spectrometry, was used to quantify protein levels in the bile of cholangiocarcinoma and control patients. The article states “Cleanascite™ (Biotech Support Group, USA), lipid removal reagent, was added to the bile and the sample was vertically shaken for 1 h at 4°C before centrifugation at 10,000 × g for 1 min.”. The authors concluded that in all, 63 proteins were significantly increased in cholangiocarcinoma bile compared to normal bile. Alpha-1-antitrypsin was one of the overexpressed proteins that increased in cholangiocarcinoma bile samples. Fecal enzyme-linked immunosorbent assay showed that alpha-1-antitrypsin level was able to distinguish cholangiocarcinoma patients from normal individuals, thereby making Alpha-1-antitrypsin a potential marker for early diagnosis of cholangiocarcinoma. “This reference shows the versatility of Cleanascite™ to efficiently remove lipids from bile fluid, with a selectivity profile suitable for proteome analysis using isobaric labels and LC-MS/MS reporting features.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Vesterhus, Mette, Anders Holm, Johannes Roksund Hov, Ståle Nygård, Erik Schrumpf, Espen Melum, Liv Wenche Thorbjørnsen et al. " Novel serum and bile protein markers predict primary sclerosing cholangitis disease severity and prognosis." Journal of Hepatology (2017). In brief, the article’s authors aimed to identify novel protein biomarkers of disease severity and prognosis in primary sclerosing cholangitis (PSC). They analyzed bile samples using a bead-based array targeting 63 proteins. The article states “The samples were thawed and 100 μL of bile was added to 150 μL PBS containing 0.1% Tween 20 and Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, Missouri, USA), then centrifuged at 4 °C for 10 minutes at 14000 rpm. CleanAscite™ (100 μL, Biotech Support Group, NJ, USA)…”. The authors identified novel biliary and serum biomarkers of severity and prognosis in PSC. Megger, Dominik A., et al. " One Sample, One Shot-Evaluation of sample preparation protocols for the mass spectrometric proteome analysis of human bile fluid without extensive fractionation." Journal of Proteomics (2016). In brief, the article’s authors report methods to overcome the biological variability of analyzing a high number of bile samples. They advance that easy sample preparation protocols are demanded representing a compromise between proteome coverage and simplicity in this study. For this, they evaluated the performance of simple workflows allowing for "one sample, one shot" experiments to identify biomarker candidates for various diseases of the hepatobiliary system. In detail, sixteen different protocols with modifications at the stages of desalting, delipidation, deglycosylation and tryptic digestion were examined. The article states “For delipidation, the Cleanascite™ Lipid Removal Reagent and Clarification Kit (BSG, NJ 08852, USA) was used following manufacturer's instructions.”. The authors concluded that delipidation yielded a considerable number of complementary protein identifications and that Cleanascite™ treatment was indispensable for in-solution digestion methods.
Danese, Elisa, et al. "
Assessment of bile and serum mucin5AC in cholangiocarcinoma: Diagnostic performance and biologic significance." Surgery (2014).
MONMOUTH JUNCTION, NJ -- Cholangiocarcinoma (CCA) is the malignant spread of biliary tree epithelial cells. Growth of cholangiocytes could be caused by primary sclerosing cholangitis, liver fluke clonorchis sinensis, hepatolithiasis, hepatitis C virus infection etc. Lipid clarification is essential on liver bile samples containing lipids, cholesterol, phospholipids. Proper sample preparation using Biotech Support Group’s Cleanascite™ could identify biomarkers of cancer in bile and serum containing samples. Authors Danese et al describe the role of mucin5AC on bile samples of cancerous and non-cancerous patients. Biliary tract tumors, cholangiocarcinoma tissues, bile and serum samples express mucin 5AC (MUC5AC) glycoprotein. An enzyme-linked immunosorbent assay was performed to obtain MUC5AC quantification from bile and serum samples of extrahepatic cholangiocarcinoma and benign biliary diseases. MUC5AC expression as a serum/bile ratio was used to differentiate cholangiocarcinoma from cholangitis, cholangiocarcinoma from biliary stones and cholangitis from biliary stones.
The article quotes “Delipidation was performed as follows: After centrifugation, the supernatant of each sample was mixed with 250 μL of Cleanascite (v/v ratio Cleanascite per sample = 1:4) and kept under mild agitation at 4°C for 1 hour to increase the agglomeration of fine lipids.” The citation is:
Danese, Elisa, et al. "Assessment of bile and serum mucin5AC in cholangiocarcinoma: Diagnostic performance and biologic significance." Surgery (2014).
Farina, Annarita, et al. "
Bile carcinoembryonic cell adhesion molecule 6 (CEAM6) as a biomarker of malignant biliary stenoses." Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics (2013).
Cancer biomarkers allow differentiating malignant from nonmalignant biliary stenoses from bile samples via comparative proteomic analysis of bile. Authors Farina et al published an article in the journal Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics titled, 'Bile carcinoembryonic cell adhesion molecule 6 (CEAM6) as a biomarker of malignant biliary stenoses' which cites Cleanascite™ from Biotech Support Group to delipidate bile samples. Bile samples were centrifuged and the supernatant was delipided with Cleanascite™ followed by ultrafiltration. Cleanascite™'s protocol is compatible with samples in which the actual lipid concentration in biological samples varies and different sample volumes are easily scaled. The reagent is a solid-phase, non-ionic adsorbent supplied as a suspension in saline, ready for use. Simply add, centrifuge and/or filter. The clarified supernatant is ready for subsequent downstream processing or analysis. Comparative proteomic biomarker discovery experiments from bile samples of malignant or benign biliary stenosis identified 66 proteins and 7 proteins were elevated in malignant/nonmalignant disease. A cell surface protein, carcinoembryonic cell adhesion molecule 6 (CEAM6), which is associated with cancer was identified via immunoblot. ELISA confirmed CEAM6 as a clinically relevant cancer biomarker of biliary cancers.
For detailed protocols of the published article click
http://www.sciencedirect.com/science/article/pii/S1570963913002410
Wang W, Ai KX, Yuan Z, Huang XY, Zhang HZ.
Different Expression of S100A8 in Malignant and Benign Gallbladder Digestive diseases and sciences. 2012; DOI: 10.1007/s10620-012-2307-0 [epub ahead of print]
Authors Wang et al published an article titled, “Different Expression of S100A8 in Malignant and Benign Gall Bladder Diseases in the journal Digestive Diseases and Sciences on discovering S100A8 as a biomarker for gall bladder cancer. Cancerous and benign analysis of human bile requires analysis of biliary protein content to find biomarkers for early diagnosis of neoplasms, pancreatic cancer, cholangiocarcinoma. In this article, scientists used Cleanascite™ from Biotech Support Group to clarify, purify and remove lipids, cell debris, lipoproteins, floating fats, impurities etc from bile samples. Bile contains high amounts of substances which interfere with protein separation and comparative analysis of bile samples. Peptides from bile samples of patients with chronic calculous cholecystitis, gall bladder cancer, gall bladder adenomas were separated by two-dimensional liquid chromatography and identified by tandem mass spectrometry. The study results from the published article identified 544, 221, and 495 unique proteins from gallbladder adenoma, chronic calculous cholecystitis, gallbladder cancer bile samples. Of the three groups of 544,221, 495 proteins one or more unique peptides were identified in 43, 16, and 28 proteins respectively. S100A8 was identified as being overexpressed in 30 proteins of gallbladder cancer bile samples as compared with benign gallbladder diseases. Unique proteins were identified and S100A8 was elevated in malignant gall bladder bile and cancerous tissues of tumor infiltrated immune cells. This study establishes Cleanascite™ as a unique delipidation and sample preparation reagent. In this study authors Wang et al concluded, “Compared with benign gallbladder diseases, consistently elevated S100A8 levels in malignant gallbladder bile and tissue indicate that gallbladder cancer is an inflammation-associated cancer. S100A8 may be a biomarker for gallbladder cancer.”
Hauser-Davis RA, Lima AA, Ziolli RL, Campos RC.
First-time report of metalloproteinases in fish bile and their potential as bioindicators regarding environmental contamination. Aquatic Toxicology.2012;110-111:99-106
Authors RA Hauser-Davis and team cites Cleanascite™ as an ideal lipid clarification reagent during sample preparation of fish bile containing matrix metalloproteinases (MMPs). The paper titled, “First-time report of metalloproteinases in fish bile and their potential as bioindicators regarding environmental contamination” is published in the journal Aquatic Toxicology.2012 Apr; 110-111:99-106. Scientists identified matrix metalloproteinases in the bile of mullets (Mugil liza) and tilapias (Tilapia rendalli) which required clarification and purification studies prior to performing SDS-PAGE and zymography analysis. Lipid removal was performed using the delipidizer Cleanascite™, which is a non-ionic adsorbent, used to precipitate lipid fat droplets, cell debris and mucinous impurities. It is ideal for clarifying ascites, serum, cell & tissue culture, bile and organ homogenates.
Farina A, Dumonceau JM, Frossard JL.
Proteomic Analysis of Human Bile from Malignant Biliary Stenosis Induced by Pancreatic Cancer Journal of Proteome Research.2009; 8(1):159-69
Using Cleanascite™ scientists isolated and identified hydrophobic polypeptides in human bile and subsequently performed specialized reversed-phase chromatography and gel-filtration, and MALDI-TOF mass spectrometry, to identify a small subset of five proteins. Bile fluid was obtained by endoscopic retrograde cholangiopancreatography (ERCP) from a patient with cholangiocarcinoma. Unfractionated bile fluid was centrifuged and partially cleared supernatant was then mixed with 250 μl of Cleanascite™ followed by rotation, centrifuged, clear away the formed lipid-micelles.
Chen B, Dong JQ, Chen YJ et al
Two-dimensional electrophoresis for comparative proteomic analysis of human bile. Hepatobiliary & pancreatic diseases international.2007 Aug;6(4):402-6
A reliable method for general clean-up of bile fluid samples, which is suitable for 2-DE, by which we built up 2-D. Bile fluid samples were obtained during surgical drainage procedures. For sample delipidation and purification, Cleanascite™ from Biotech Support Group was used to remove debris, nucleic acid and mucins followed by rotation for 1 hour. Salts, lipids, nucleic acids and other contaminants, are bound in bile fluid, dramatically affects both reproducibility and resolution of 2-DE. The objectives of our study were to establish a reliable sample preparation method and 2-DE options suitable for comparative proteomic analysis of bile fluid.
Guerrier L, Claverol S, Finzi L et al
Contribution of solid-phase hexapeptide ligand libraries to the repertoire of human bile proteins. Journal of Chromatography A.2007;1176(1-2):192-205
Scientists used Immobilized peptide ligand libraries to concentrate dilute bile. For the detection of low abundance proteins from bile requires Cleanascite™ was used to clarify bile. Cleanascite™ selectively removes lipids, cell debris, lipoproteins, floating fats, impurities from cohn paste, transgenic milk, egg yolk and biological samples for pretreatment of samples prior to purification. The reagent is a solid-phase, non-ionic adsorbent supplied as a suspension in saline, ready for use. Pre-treatment with hexapeptide ligand librairies opens up new perspectives in the discovery of biomarkers in human bile.
Chen Bo, Zheng Jian-wei, Wang Jian-ming, et al.
Establishment and preliminary analysis of a 2-D human biliary map Chinese Journal of Hepatobiliary Surgery.2007
Surgical drainage of bile fluid samples from patients with cholangiocarcinoma and cholelithiasis was collected, sonicated and centrifuged to remove debris, nucleic acid and mucins as a preliminary separation. Cleanascite™ was used for sample delipidation allowing for proper sample preparation process suitable for two-dimensional electrophoresis of bile fluid. By doing so, bile fluid analysis and identification of biomarkers by 2-D biliary maps are visualized.
Kristiansen TZ, Bunkenborg J, Gronborg M et al
A Proteomic Analysis of Human Bile Molecular and Cellular Proteomics.2004;3:715-728
Scientists discovered large amounts of lipids, bile salts in bile fluid obtained by ERCP from patients with cholangiocarcinoma. To identify proteins in bile fractions researchers used Cleanascite™ to precipitate lipids from “unfractionated bile” followed by one-dimensional gel electrophoresis, lectin affinity chromatography and liquid chromatography tandem mass spectrometry. Cleanascite™ successfully precipitate lipids, fat droplets, cell debris, and mucinous impurities. 3-kDa size-exclusion filtration step subsequently removed salts.
Egg Yolk
Ben Wade, Michelle Cummins, Anthony Keyburn and Tamsyn M. Crowley. Isolation and detection of microRNA from the egg of chickens. BMC Research Notes 2016 9:283.
MONMOUTH JUNCTION, NJ, June 1, 2016 -- Biotech Support Group reports on a recent research article describing the simplicity and efficiency of their proteomic sample preparation technology for clearing lipids from egg yolk. The citation is: Ben Wade, Michelle Cummins, Anthony Keyburn and Tamsyn M. Crowley. Isolation and detection of microRNA from the egg of chickens. BMC Research Notes 2016 9:283.
DOI:
10.1186/s13104-016-2084-5 “This is still another example of the versatility of Cleanascite™ as it now shown to help purify microRNA from egg yolk, a very lipid-rich tissue type. The exquisite selectivity profile of Cleanascite™ makes this possible.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Organ Homogenates
Myerson, J., He, L., Lanza, G., Tollefsen, D. and Wickline, S.
Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. Journal of Thrombosis and Haemostasis.2011;9:1292-1300
Cleanascite™ was used for lipid removal in an article published in the Journal of Thrombosis and Haemostaisis. For the localized control of acute thrombosis, scientists have developed a new platform that uses PPACK (Phe[D]-Pro-Arg-Chloromethylketone) with nanoparticles that serves as thrombin-inhibiting surfaces at sites of acutely forming thrombi to prevent the effects of local clot inhibition. A new article published on thrombin presents a thrombin inhibitor complexed with a nanoparticle as a anticoagulant with a multivalent thrombin adsorbing particle surface. In this article, PPACK and PPACK nanoparticle inhibition of thrombin were assessed in vitro via thrombin activity against a chromogenic substrate. Also in vivo acute arterial thrombosis model demonstrated that PPACK nanoparticles outperformed both heparin and uncomplexed PPACK in inhibiting thrombosis. So overall this article shows how Cleanascite™ is used for sample preparation during experiments in vascular diseases to develop new products such as thrombin-inhibiting perfluorocarbon nanoparticles that may novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. The article mentions how Cleanascite™ is used: "The extent of PPACK coupling was determined by reverse-phase HPLC quantification f uncoupled PPACK after centrifugation of nanoparticles with Cleanascite™ lipid adsorption reagent from Biotech Support Group."
Thakuria D, Schmidt O, Liliensiek AK.
Field preservation and DNA extraction methods for intestinal microbial diversity analysis in earthworms. Journal of Microbiological Methods.2009;76(3):226-33
Endothelial nitric oxide synthase (eNOS) catalyzes the production of the multifunctional second messenger nitric oxide (NO), l-citrulline from l-arginine, O2, and NADPH-derived electrons. It plays an important role in cerebro-vasoregulatory mechanisms in hypotension and ischemia. Quantitative measurement of proteins from brain tissue from eNOS using enzyme-linked immunosorbent assay (ELISA) technique requires reducing matrix effects. Matrix effects interfere with the detection of the analyte because of brain lipids, lipoproteins, proteins. To reduce assay interference by lipids Cleanascite™ was used for lipid removal and clarification. The simple protocol involves adding Cleanascite™ (200 μL) to rat brain tissue homogenate (800 μL) and mixing for 20 min at room temperature. Finally sample was centrifuged (1000g) for 20 min at 4 °C and the supernatant analyzed.
R. Kenneth Czambel, Alexander Kharlamov, Stephen C. Jones,
Variations of brain endothelial nitric oxide synthase concentration in rat and mouse cortex, Nitric Oxide, Volume 22, Issue 1, 1 January 2010, Pages 51-57
To assess possible assay interference by lipids present in the homogenate matrix, samples were treated with Cleanascite™ (Biotech Support Group, North Brunswick, NJ), a commercially available lipid removal and clarification reagent. This reagent is a saline suspension of a solid-phase non-ionic adsorbent (pH 8.0) that selectively removes lipids from biological samples. Immediately prior to use, the Cleanascite™ reagent was completely resuspended by gentle shaking. Cleanascite™ (200 μL) was added to rat brain tissue homogenate (800 μL) and mixed for 20 min at room temperature by gentle shaking. Following centrifugation (1000g) for 20 min at 4 °C, the supernatant was carefully decanted into a clean collection vial and analyzed.
Cheng AM, Moore EE, Masuno T et al
Normal Mesenteric Lymph Blunts the Pulmonary Inflammatory Response to Endotoxin. Journal of Surgical Research.2006;136(S2):166-171
LPS induced ICAM-1 expression decreases by lipoproteins in normal mesenteric lymph(NML) which contain anti-inflammatory factors. Cleanascite™ was used for delipidation and removal of lipoproteins from primary human pulmonary endothelial cells (HMVECs) incubated with normal mesenteric lymph NML or post-shock mesenteric lymph PSML. ICAM expression was measured after LPS stimulation by flow cytometry. ICAM-1 surface expression was measured by flow cytometry. Cleanascite™ extracted lipoproteins from NML before incubation and LPS-induced ICAM-1 expression was determined. Researchers concluded that decreased lipoprotein expression after hemorrhagic shock HS increases post-shock mesenteric lymph PSML toxicity from the ischemic gut.
McNally T, Mackie IJ, Machin SJ et al.
Increased levels of beta 2 glycoprotein I antigen and beta 2 glycoprotein I binding antibodies are associated with a history of thromboembolic complications in patients with SLE and primary antiphospholipid syndrome British journal of rheumatology.1995 Nov;34(11):1031-6
Scientists measured β2GPI antigen (β2GPI: Ag), β2GPI aPA cofactor activity (β2GPI: Cof) and antibodies to β2GPI (αβ2GPI) from systemic lupus erythematosus (SLE) patients with aPAs (SLE-aPA +) and primary antiphospholipid syndrome (PaPS). Researchers implemented the Cleanascite protocol for studying β2 Glycoprotein-I (β2GPI), a cofactor for binding antiphospholipid antibodies and with with in vitro anticoagulant properties in plasma. After lipids, cell debris, lipoproteins, floating fats, cohn paste, transgenic milk, egg yolk and mucinous impurities from biological samples are removed via Cleanasicte™’s unique technology, measuring cellular function and detection of cellular mediators is possible. Cleanascite™ from Biotech Support Group offers a rapid implementation method with reproducible and consistent results that ensure clarity of samples and compatibility of downstream processing.
Template Preparation - Production Sequencing Protocols used at the Stanford Genome Technology Center
During template preparation yeast inserts are cloned into M13 vectors followed by amplification and isolation of DNA. Cleanup of DNA follows by adding 60 µl of Cleanascite™ to wells containing the phage/insert DNA. Next, shaking, centrifugation, and transferring supernatant to 96-well filter plate occurs. Additional centrifugation, washing with ethanol (for DNA precipitation) followed by centrifugation isolates DNA. Yield of DNA varied based on initial pellet quantity.
Red Blood Cells
Antunes RF; Brandao C; Maia M; Arosa FA.
Red blood cells release factors with growth and survival bioactivities for normal and leukemic T cells. Immunology and Cell Biology.2011;89(1):111-21
Authors Antunes et al research titled, “Red blood cells release factors with growth and survival bioactivities for normal and leukemic T cells” examined why red blood cells might prevent progression of malignant cell growth in vivo thru bioactive factors which increase proliferation of activated T cells and releases leukemic Jurkat T cell( erythrocyte-derived growth and survival factors). In vitro culture of human RBC spontaneously released protein factors that enhance T-cell growth and survival of normal and malignant activated T cells. RBC-CM generated from cultures of RBC reproduces the effectiveness of intact RBC in modulating proliferation, cell growth and survival of activated T cells. Often Sudan black staining does not detect lipids in the RBC-sup. Excessive lipids affect LC-MS results. For eliminating the possibility of lipid molecules which might be responsible for the bioactivity of red blood cells factors, researchers used Cleanascite™ in the protocol. Following ultrafiltration and concentration of the in vitro assay of RBC-sup was quantified. For thermostability studies, the RBC-sup was boiled, concentrated and centrifuged preparing it for in vitro bioactivity assays. Scientists used Cleanascite™ for lipid depletion from the RBC-sup. Cleanascite™ was used in a ratio 1:4, incubated, centrifuged, collected and concentrated according to the protocol from Biotech Support Group for preparing the RBC-sup for vitro bioactivity assays.
Tracheal swab samples
Li D, Wang J, Wang R, Li Y.
A nanobeads amplified QCM immunosensor for the detection of avian influenza virus H5N1, Biosensors and Bioelectronics.2011;26(S10):4146-4154
Magnetic nanobeads amplification method based quartz crystal microbalance (QCM) immunosensor was tested for AI H5N1 virus detection. Captured H5N1 viruses by immobilized antibodies are measured by changes in frequency. Researchers used Cleanascite for selectively removing lipids, cell debris, lipoproteins, floating fats, impurities for pretreatment of samples prior to purification from tracheal swab samples for efficiently detecting pathogenic avian influenza (AI) H5N1 virus.
Fu LM, Shinnick TM.
Genome-wide analysis of intergenic regions of mycobacterium tuberculosis H37Rv using affymetrix genechips. EURASIP journal on bioinformatics & systems biology.2007:23054
Researchers from the Pacific Tuberculosis and Cancer Research Organization and Centers for Disease Control and Prevention used Cleanascite during sample preparation for sequencing the complete genome of Mycobacterium tuberculosis H37Rv. To explore potential protein-coding genes, scientists reviewed searched for the gene structure, protein coding potential, and presence of ortholog evidence. After performing bacterial lysis and RNA isolation, Cleanascite was used on the recovered aqueous phase for removes lipids, cell debris, lipoproteins, floating fats, impurities from cohn paste, transgenic milk, egg yolk and biological samples for pretreatment of samples prior to purification. The RNA sample was precipitated and purity measured by UV.
Tissue and Cell Culture
United States Patent Application 20170348398 entitled: "COMPOSITIONS AND METHODS FOR DECREASING BLOOD GLUCAGON LEVELS”
Biotech Support Group reports on a recent patent application describing the simplicity and efficiency of their lipid clearance sample preparation technology for evaluating the influence of lipids on pancreatic islet culture. The patent discloses compositions and methods for decreasing blood glucagon levels. As disclosed, L-glutamine is a selective stimulator of α-cell proliferation generated when glucagon signaling is interrupted. A method for treating a subject with hyperglucagonemia, e.g., a subject with diabetes, that involves administering to the subject a composition comprising an L-glutamine inhibitor in an amount effective to decrease blood glucagon levels, is disclosed. In an example, pancreatic islets were isolated from male 8-14 week old C57B16/J mice (Jackson Laboratory, ME) and cultured in various media conditions for 3 days. The patent states “For lipid removal, serum was treated with Cleanascite reagent (Biotech Support Group, Monmouth Junction, N.J.) prior to islet culture at a 1:1 ratio according to the vendor's protocol.”. The example supports that increased amino acids, but not lipids and other soluble factors, selectively increased rapamycin-sensitive α-cell proliferation. “This reference shows the versatility of Cleanascite™ to efficiently remove lipids from serum tissue culture, so that factors that influence cell proliferation can be investigated.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Ahmed, Syed Mukhtar, and Ian G. Macara.
The Par3 polarity protein is an exocyst receptor essential for mammary cell survival. Nature Communications.8 (2017): 14867. doi:10.1038/ncomms14867
In brief, the article’s authors aimed to identify a receptor of the exocyst, an essential component of the secretory pathway required for delivery of basolateral proteins to the plasma membranes of epithelial cells. To determine if phospholipids were essential for the interaction, a lipid-binding resin was used to remove lipids. The article states “Lipids from cells and bacterial lysates were removed using Cleanascite™…”. The authors conclude that Par3 is an exocyst receptor required for targeted membrane- protein delivery.
Alhamdani MS, Schroder C, Hoheisel JD.
Analysis conditions for proteomic profiling of mammalian tissue and cell extracts with antibody microarrays. Proteomics.2010;10(17):3203-7
As a lipid removal reagent, Cleanascite™ was used to purify seven separate pancreatic cancer tissue samples which contained high lipid content. The researchers found that the sample delipidation provided by Cleanascite™ was necessary for studying the tissue homogenates. Cleanascite™ was able to substantially improve the array quality of the pancreatic cancer tissue samples. Scientists from the Department of Neurology, Allegheny-Singer Research Institute in Pittsburg and Department of Radiology at University of Pittsburgh have published a paper in the journal, Nitric Oxide, titled "Variations of brain endothelial nitric oxide synthase concentration in rat and mouse cortex" by Czambel RK,et al to assess for assay interference by lipids present in the homogenate matrix while studying brain cortex samples.
Plasma-Enzyme-Nanoparticle Mixture
Pan, Dipanjan, et al. "
Anti-Angiogenesis Therapy in the Vx2 Rabbit Cancer Model with a Lipase-cleavable Sn 2 Taxane Phospholipid Prodrug using αvβ3-Targeted Theranostic Nanoparticles." Theranostics 2014; 4(6):565-578
Authors Pan et al published an article in the journal Theranostics titled, Anti-Angiogenesis Therapy in the Vx2 Rabbit Cancer Model with a Lipase-cleavable Sn 2 Taxane Phospholipid Prodrug using αvβ3-Targeted Theranostic Nanoparticles', describing nanoparticle formulations for anti-cancer chemotherapy. Research involved developing and characterizing a Sn 2 lipase-labile prodrug of docetaxel Dxtl-PD. Excessive serum and lipase surrounding taxane prodrug in PFC nanoparticles was optimized using Cleanascite™, a lipid removal reagent. In vitro experiment on anti-angiogenic molecule docetaxel prodrug in perfluorocarbon (PFC) nanoparticles cites Cleanascite™ for lipid removal from plasma-enzyme-nanoparticle mixture. Excessive lipids in samples interferes in high performance liquid chromatography. Subsequent to sample pretreatment by implementing the Cleanascite™ protocol, the mixture is centrifuged and supernatant is analyzed by HPLC. Less matrix effects are observed in high performance liquid chromatography analysis as Cleanascite™ removes lipids and does not bind to DNA, RNA, enzymes and proteins in samples. Next, 2-propranol and water was added to red blood cell aliquots and mixture was centrifuged. The RBC fraction is separated from PFC nanoparticles.
Saliva
Lucy E. DesJardin
Isolation of M. tuberculosis RNA from Sputum Methods in Molecular Medicine.2001;48:133-139
Cleanascite™ was used for RNA isolation from M.tuberculosis(MTB) samples. MTB mRNA are frequently analyzed for chemotherapy efficacy. Measuring acid fast bacilli positive strains and positive sputum culture conversion to negative before and after chemotherapy, allows scientists to measure the bactericidal effect and develop innovations in design of clinical trials for new treatments. Producing MTB RNA from small volume of sputum requires isolating RNA. Cleanascite™ was added to the aqueous phase of the sample, followed by centrifugation and supernatant removal.
Beenhouwer DO, Shapiro S, Feldmesser M et al.
Both Th1 and Th2 Cytokines Affect the Ability of Monoclonal Antibodies To Protect Mice against Cryptococcus neoformans Infection and immunity.2001;69: 6445-6455
Scientists analyzed impact of passively administered IgG subclasses to mice deficient in Th1 cytokine interleukin-12 (IL-12), the proinflammatory cytokine IL-6, or the Th2 cytokines IL-4 and IL-10 against cryptococcal infection. In the study variable-region-identical IgG1, IgG2a, IgG2b, and IgG3 monoclonal antibodies were analyzed against intravenous infection withC. neoformans in mice genetically deficient in interleukin-12 (IL-12), IL-6, IL-4, or IL-10. For the purification of monoclonal antibodies, IgG3 hybridoma and IgG1, IgG2b, IgG2a switch variants of MAb 3E5 developed from in vitro isotype switching were injected with ascites fluid from hybridoma cells. Cleanascite™ was successfully used for removing lipids and cell debris and the ascites fluid was sterilized. The antibody concentration was measured by enzyme-linked immunosorbent assay (ELISA).
Desjardin LE, Perkins MD, Wolski K et al.
Measurement of Sputum Mycobacterium tuberculosis Messenger RNA as a Surrogate for Response to Chemotherapy American journal of respiratory and critical care medicine.1999;160(1):203-10
Authors of this article isolated mycobacterial RNA from specimens developing molecular markers for quantification of mRNA as a response for monitoring response to chemotherapy and for assessing the efficacy of new drugs for suspected multidrug-resistant tuberculosis. Levels of a stable and abundant structural RNA, 16S rRNA are isolated and added to homogenized sputum into a matrix tube for cell. After spinning and processing, 200 µl of chloroform was added and the aqueous and organic layers were separated by microcentrifugation. The aqueous phase which has the RNA was removed and 100 µl Cleanascite™ is used to purify the sample. The aqueous phase is extracted with 500 µl chloroform:isoamyl alcohol and RNA is precipitated extracted with phenol and CHCl3, precipitated with isopropanol, and resuspended in a final volume.
Patents
Shiffman, Dov, et al. "Methods for quantitation of insulin and c-peptide." U.S. Patent Application No. 15/942,188.
US Patent 20130011413:
Method and Pharmaceutical Composition for Treatment of Intestinal Disease
Inventors Iwakura Yoichiro, Kakuta Shigeru and Suzuki, Shunsuke and assignee The University of Tokyo published United States Patent Application 20130011413 titled, Method and Pharmaceutical Composition for Treatment of Intestinal Disease. The patient details a method for using interleukin-related substances like an IL-17F inhibitor typified by an anti-IL-17F antibody to treat intestinal disease such as colon polyps or colorectal cancer. The patent cites Cleanascite™ from Biotech Support Group in a method for the selection of neutralizing antibodies to mouse IL-17F and IL-17A. Neutralizing antibodies anti-IL-17F antibody and anti-IL-17A antibodies selected were cultured in serum-free medium and from the supernatant purified antibody was developed. The hybridoma culture supernatant was recovered periodically and a one fourth quantity of Cleanascite (registered trademark) (Biotech Support Group, LLC) was added to remove lipid and lipid like material from the recovered culture supernatant. Next at room temperature the samples were shaked for 10 minutes followed by centrifugation at 2000rpm to recover the supernatant. Subsequently, filtering, purification, elution, dialysis, filter sterilizing was followed on the samples and protein concentration and the degree of purification was confirmed.
J Krupey - United States Patent: 5885921
Hydrophobic silica adsorbents for lipids
Researchers cite Cleanascite™ for lipid removal and sample clarification of viruses from biological fluids, in bioprocessing, clarifying ascites fluid and tissue culture and for removing colloidal lipid and cell debris from bacteria and yeast homogenates. Cleanascite™ complements isolation and analysis of hemoglobin from blood products by removing lipids and lipoproteins from whole blood and serum. Cleanascite™ has high affinity and specificity for lipids and simultaneou low affinity for proteins allowing researchers to prepare samples efficiently.
David C. Jones. United States Patent: 7999084.
Devices and methods for reducing matrix effects
Authors cite Cleanascite’s lipid removal properties for decreasing interference from analytes during bioanalytical testing and quantitation. For example matrix effects often cause analytical errors for liquid chromatography-mass spectrometry/mass spectrometry (LC/MS-MS) during drug metabolism studies(Little, J. L. et al. (2006) J. Chromatog. 833, 219). Phospholipids such as phosphatidylcholines interfere with analyte ionization in electrospray MS detection by reducing analyte sensitivity referred to as ion suppression or matrix effects. Cleanascite™ from Biotech Support Group is cited in U.S patent application 20110263040 titled, “Devices and Methods For Reducing Matrix Effects”. Cleanascite™ is used for selectively removing lipids, cell debris, lipoproteins, floating fats, impurities for pretreatment of samples prior to purification. Bioanalysis is analyzing (using LC-MS or HPLC) drug/metabolites/biomarkers (qualitative/ quantitative) in biological samples such as plasma, serum, whole blood, urine, saliva, tissues, etc. The matrix effect is the ion-suppression and/or enhancement observed in electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI)during LC-MS use. Such interference coming from biological matrix is reduced with Cleanascite sample preparation reagent. Pretreatment of samples reduces interferences of contaminants and increases accuracy of analytes during bioanalytical testing and quantitation methods such as liquid chromatography-mass spectrometry/mass spectrometry. For example, matrix effects or ion suppression caused by phospholipids (phosphatidylcholines) in mass spectrometry results in low recovery and high variation of results. This patent cites U.S. Pat. No. 5,885,921 to Krupey which describes the use of Cleanascite, the hydrophobic silica adsorbents for the removal of lipids in samples. Thus Cleanascite provides a rapid procedure for the removal of phospholipids and interfering analytes causing matrix effects and proteins from a bioanalytical sample prior to performance of analytical procedures.
Morre, James D et al. United States Patent: 20030170757.
Monoclonal antibodies specific for neoplasia-specific NADH: disulfide reductase
Researchers used Cleanascite™ for delipidation of sera followed by proteinase K treatment to determine cancer specific bands from samples used for developing monoclonal antibodies which specifically recognize the tumor cell specific NADH:protein thiol reductase and hybridoma cell lines. SDS Page and Western blot analysis of sera from cancer patients (with prostate, lymphoma, ovarian, leukemia, breast) and health patients followed.
Mcintyre, John A. United States Patent: 20120107841.
Serum Diagnostic Method, Biomarker and Kit for Early Detection and Staging of Alzheimer's Disease
Cleanascite™ for Sample Preparation Of Hemin Treated Serum Samples and Redox-Reactive Anti-Phospholipid Autoantibodies: Scientists are actively searching for serological biomarkers of Alzheimer's disease (AD) resulting in early diagnosis and staging of AD. Blood tests which measure redox reactive autoantibodies before and after exposure to an oxidative agent in serum for the presence of an elevated level of redox-reactive autoantibodies are being researched by scientists for developing a laboratory method for screening, diagnosing, monitoring and/or staging early onset Alzheimer's disease.Scientists are researching redox-reactive autoantibodies (R-RAA) present in serum which unmask antigen recognition sites upon oxidative exposure. Using ELISA to quantitatively measure concentration of unmasked antibodies in serum samples in vitro by the presence of their recognition epitopes, scientists could assess the increase in R-RAA over their base line values and compare it to the increased ELISA reactivity in AD and/or normal individuals. Comparisons between the AD and normal populations revealed highly significant differences in R-RAA antiphosphatidylethanolamine (aPE). Essentially the autoantibodies are detected in blood with an oxidizing agent and then using a screening assay to detect antibodies that bind a self antigen. Hemin is the oxidizing agent used for R-RAA aPE ELISA. Inventors John Mcintyre developed technology to ‘unmask’ these autoantibodies in serum samples in vitro and matched AD specific epitopes to reactive autoantibodies. The increase in R-RAA aPE in MCI serum samples and changes in hippocampal choline acetyltransferase (ChAT) in end-stage AD or MCI are being exploited as potential biomarkers.United States Patent Application 20120107841 titled, “Serum Diagnostic Method, Biomarker and Kit for Early Detection and Staging of Alzheimer's Disease” cites Cleanascite™ for lipid removal and clarification. For the preparation of serum samples, Cleanascite™ from Biotech Support Group was added. Specifically 1:4 vol/vol of Cleanascite™ is added to each serum sample in a 2 ml micro tube, using a pipette tip.
Vaccine Research (Cholesterol Removal From Human Serum)
Kamtchoua, Thierry, Monica Bologa, Robert Hopfer, David Neveu, Branda Hu, Xiaohua Sheng, Nicolas Corde, Catherine Pouzet, Gloria Zimmerman, and Sanjay Gurunathan.
Safety and immunogenicity of the pneumococcal pneumolysin derivative PlyD1 in a single-antigen protein vaccine candidate in adults. Vaccine (2012).
Authors Thierry Kamtchoua et al published an article in the journal Vaccine titled, ‘Safety and immunogenicity of the pneumococcal pneumolysin derivative PlyD1 in a single-antigen protein vaccine candidate in adults’ describing the immunogenicity of pneumococcal single antigen protein vaccine in a phase 1, randomized, placebo controlled dose escalating study. Authors cite Cleanascite™ from Biotech Support Group for removal of cholesterol from serum. A toxin neutralizing assay with antibodies in sera was developed to neutralize cytotoxicity caused by Ply in Vero cells. An incubated challenge dose of pneumolysin toxin containing serum diluted with or without CleanasciteTM was developed. The neutralizing titer inhibited the toxin’s effect on Vero cells. According to the paper, “Briefly, the toxin-neutralizing antibody titer was determined by incubating a challenge dose of pneumolysin toxin with serial 2-fold dilutions of serum treated with or without Cleanascite™ HC (Biotech Support Group) to remove cholesterol, an inhibitor of Ply”.
Yeast assays
Lifang, et al.
Balanced globin protein expression and heme biosynthesis improve production of human hemoglobin in Saccharomyces cerevisiae. Metabolic Engineering (2013).
For hemoglobin assays it is necessary to remove lipids. Authors Liu et al used lipid removal reagent Cleanascite™ in an article titled, Balanced globin protein expression and heme biosynthesis improves production of human hemoglobin in Saccharomyces cerevisiae. Lipid removal from yeast and blood samples for hemoglobin production is an important component of proteomics research. Researchers are increasingly interested in the production of recombinant human hemoglobin from Escherichia coli and yeast hemoglobin which maintains current good manufacturing practices. Sample preparation provides effective methods for enriching and depleting hemoglobin from complex samples. Subsequent to sample preparation the samples produced maintain hemoglobin stability, mitigate iron-catalyzed reactions, and hemin production as well as expression and purification protocols are of high quality. Authors Liu et al published an article in the journal Metabolic Engineering and the article cites lipid removal agent Cleanascite™ for sample clarification of hemoglobin assays. The article describes experiments on SDS-PAGE with heme and globin expression, globin patterns, heme, corproporphyrin and porphyrin levels, batch fermentations with heme and hemoglobin production levels The article quotes "Lipid removal agent Cleanascite (X2555-100, BIOTECH SUPPORT GROUP) was added according to product description in case of unclear suspensions in the supernatant. Protein concentration was measured by BCA protein assay kit".
Human & Plasma Cell Lines
Pan, Dipanjan, et al. "
A strategy for combating melanoma with oncogenic c-Myc inhibitors and targeted nanotherapy." Nanomedicine 10.2 (2015): 241-251.
Research article in the journal NanoMedicine cites Biotech Support Group's Cleanascite™ sample preparation reagent to obtain lipid clarified c-Myc inhibitor prodrug used in a drug delivery and enzymatic release platform for melanoma antiproliferation. Samples composed of human and mouse cell lines containing Sn-2 lipase-labile Myc inhibitor prodrug on a αvβ3-targeted nanoparticle platform to deliver c-Myc inhibitors were researched as an alternative to small-molecule inhibitors of c-Myc–Max. Lipid clarification was performed on synthesized Sn-2 lipase-labile Myc inhibitor prodrug in two αvβ3-targeted nanoparticle platforms (20 and 200 nm). Subsequent nanodelivery of c-Myc inhibitors to prevent melanoma progression requires sample preparation. Melanoma antiproliferation potency of lipid containing and lipid clarified human and mouse melanoma cell lines was obtained using Biotech Support Group's Cleanascite™.
Cleanascite™ - Lipid Removal and Cell Response Applications The “omics” revolution demanded new and different sample prep separations that were not efficiently performed by conventional technologies. While effective for many applications, these tools were not efficient for “omics” sample preparation, as throughput, economy and simplicity are especially required. Furthermore, these same separation tools often denatured proteins which limited there use in applications which demanded the measurement of function, structure or bio-activity. For these reasons, BSG has been dedicated to create new methods and applications to drive efficient workflows and better data quality for all proteomic and biomarker analyses. Of special importance is the value created when certain families of biomolecules can be evaluated with respect to cell response and viability. For example, extracellular vesicles (EVs) substantially influence cultured cell behavior. While all of our products serve cell response applications, we report here an extensive list of applications in this area for Cleanascite™. Cleanascite™ is derived through a proprietary formulation of metallic oxide derivatives. However,unlike other metallic oxides, Cleanascite™ does not have significant protein binding, making its selectivity profile for lipids un-rivaled in the bio-research products industry. As a result, it is ideal to clear lipid-associated matrix effects - including extracellular vesicles, which may influence cell response assays. To download whitepaper entitled “Cleanascite™ - Lipid Removal and Cell Response Applications" Visit: http://www.biotechsupportgroup.com/v/vspfiles/templates/257/pdf/CleanasciteCellResponseReferenceApplications121218.pdf
|
|
 |
