Lipid Removal & ClarificationCleanascite™Lipid Adsorption & Clarification
Cleanascite™ is derived through a proprietary formulation of metallic oxide derivatives. Unlike other metallic oxides, Cleanascite™ does not have significant protein binding making its selectivity profile for lipids unique in the bio-research products industry. As a result, it is ideal to clear lipid-associated matrix effects from human sera, bile, ascites, and other high lipid content sample types. View Details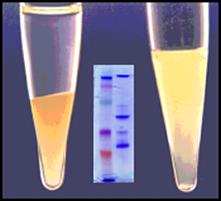
Discard
Lipids Cholesterol Extracellular Vesicles ↑
Cleanascite™ Improved Assay Performance - ELISA - Immunocapture Microarrays - LC-MS - Toxin Neutralizing Titer - Cell Response 
Cleanascite™ is supplied as a suspension reagent. Simply add, mix and centrifuge in a 10 minute protocol. Key ReferencesPlasma/Serum Protein BiomarkersThe authors aimed at simultaneously measuring intact insulin and proinsulin derived C-peptide, to help predict development of diabetes mellitus, as well as in differential diagnosis in cases of hypoglycemia. Cleanascite™ is shown both to improve LC-MS measurements, and validated in accordance with CLIA ’88 guidelines. { doi: 10.1016/j.cca.2016.01.019 } Vaccine DevelopmentTo evaluate immunogenic response to a vaccine candidate, it is necessary to measure the antibodies from sera; a sample with a diverse lipid profile. In this citation, Cleanascite™ was used in a toxin neutralizing assay to evaluate the influence of cholesterol dependency, on a candidate protein pneumococcal vaccine. { doi: 10.1016/j.vaccine.2012.11.005 } Bile ProteomicsThe authors report methods to overcome the biological variability of analyzing a high number of bile samples. They concluded that delipidation yielded a considerable number of complementary protein identifications and that Cleanascite™ treatment was indispensable for in-solution digestion methods. { http://dx.doi.org/10.1016/j.jprot.2016.11.021 } Ascites Monoclonal AntibodiesThe researchers determined the role of complement on MAb-mediated protection for four mice Ig subclasses. After centrifugation of ascetic fluid, Cleanascite™ protocol was implemented to remove lipids. { doi: 10.1128/IAI.70.5.2598-2604.2002 } Cellular Response ApplicationsThe applications and references for the many diverse investigations using Cleanascite™ upstream of cell response measurements are described. { Cleanascite Cell Response Reference Applications } Cleanascite™ LXFor Lipemic Serum/Plasma Clarification
Protocol Cleanascite™ LX is supplied as an aqueous suspension of non-ionic adsorbent in DI water, pH 8.0. After centrifugation, the pellet is 1/2 of the total volume and the supernatant is 1/2 of the total volume. View Details
Optimization. Different sample volumes are easily scaled. Volume ratio can be adjusted up or down as required to remove the amount of impurities present. Our BSG AdvantageConsumableCost-effective, not derived from biologicals
On-Bead DigestionEfficient workflows, quality LC-MS/MS data
Enrichment/DepletionDiverse strategies, species agnostic
Functional IntegrityMaintained throughout all separations
|

- About
- Products
- Hemoglobin Removal Kits
- Lipid Removal & Clarification
- Urine Protein & Low Abundance Enrichment
- Class Specific Enrichment
- Sample Prep Mass Spectrometry
- Functional & Chemical Proteomics
- Genomic Sample Prep
- Accessories
- Technical Resources
- References
- Publications & Reports
- FAQs
- Case Studies
- Cleanascite™ Unlocks Insights into Lipid-Driven Tumor Immunosuppression
- NRicher™ Bead Platform Provides Unique Sub-Proteome Biases And Fit For Purpose Opportunities for Targeted LC-MS Quantification
- BSG Products To Assist in Analyzing Macrophage Polarization
- Ectodomain Shedding and Enrichment of the Soluble Membrane Proteome
- Investigate out of the Venn Diagram box
- Methods to selectively deplete or purify Hemoglobin from Dried Blood Spots (DBS)
- The Utility of HemoVoid™ is Demonstrated in 3 Proteomic Investigations Identifying Potential Disease Specific Biomarkers
- The 4 common features of our sample prep products, known as the BSG Advantage, are highlighted in a selection of journal references.
- AlbuVoid™ Workflows Advance Cell Secretome Proteomics
- Lipid Removal for Phenotypic Cell Response in Cancer Research
- The Influence of Sample Prep Bias on LC-MS Targeted Peptide Quantification in Serum Proteomics
- Re-imagining proteomics for developing precision medicine biomarkers of the innate immune response in SARS-CoV-2
- Patent Application Describes New Proteomic Methods to Monitor Protease Inhibitor Function During Covid-19 Infections
- Efficient Hemoglobin Removal Advances Red Cell Proteomics Offering Many New Insights Into Inflammation and Infectious Disease
- The Potential for New Blood Biomarkers in the Management of COVID-19 Disease
- Establishing the Utility of HemoVoid™ and HemogloBind™ as Enrichment Tools for Proteomic Analysis of Red Cells and Whole Blood in Parkinson’s Disease
- Species Diversity Supported By BSG Products
- Poster Report Describes Loss of Functional Serpin Activity In Cancer Patient Blood
- AlbuVoid™️ PLUS & AlbuSorb™️ PLUS Evaluating Different Windows of Observation Solves The Many Challenges of Serum Proteomics
- Tackling the Challenges of Serum Proteomics
- Lipid Removal Sample Prep for Cell Response Applications
- Sample Prep for Proteomic Analysis of Saliva
- Biotech Support Group Featured in Book, "Functional Proteomics – Methods and Protocols"
- Sample Prep Liquid Biopsy Products Suitable for Proteomic Profiling of a Variety of Body Fluid Sample Types
- Albumin and High Abundance Depletion
- Using HemogloBind™ as a Hemoglobin Binding Reagent
- Diverse technologies available for researchers to selectively bind or enrich exosomes and extracellular vesicles.
- Stroma Liquid Biopsy™ Biomarkers Profile Pan-Cancer Dysregulation of the Serum Proteome
- Diverse Depletion and Enrichment Technologies Enhance Simplicity and Efficiency of Obtaining Quality Proteomic Information
- Use On-Bead Digestion to Improve Time Required for Serum Digestion
- Using AlbuVoid™ as a Serum Protein Enrichment Kit in Functional Proteomics
- Using Cleanascite™ as a Lipid Absorption and Clarification Reagent
- Using HemoVoid to Remove Hemoglobin Before Analysis
- Blog
- Contact
- Liquid Biopsy